Article Contents
| Korean J Pediatr > Volume 58(8); 2015 |
|
Abstract
Purpose
We assessed the relationships between iron and vitamin D statuses in breastfed infants and their mothers and evaluated the determinants of iron and vitamin D deficiencies in breastfed infants.
Methods
Seventy breastfed infants aged 4-24 months and their mothers participated in this study from February 2012 to May 2013. Complete blood counts, total iron binding capacity, and levels of C-reactive protein, iron, ferritin, calcium, phosphate, alkaline phosphatase, and 25-hydroxyvitamin D (25(OH)D) in infants and their mothers were measured.
Results
A history of maternal prepregnancy anemia was associated with lower ferritin and 25(OH)D levels in both infants and their mothers. The 25(OH)D level of infants correlated with maternal 25(OH) D levels. The independent risk factors for iron deficiency in breastfed infants were the duration of breastfeeding (odds ratio [OR], 6.54; 95% confidence interval [CI], 1.09-39.2; P=0.04) and infant body weight (OR, 2.65; 95% CI, 1.07-6.56; P=0.04). The determinants for vitamin D deficiency were the infant's age (OR, 0.15; 95% CI, 0.02-0.97; P=0.046) and maternal 25(OH)D level (OR, 0.74; 95% CI, 0.59-0.92; P=0.01).
Conclusion
A maternal history of prepregnancy anemia requiring iron therapy was associated with lower current ferritin and 25(OH)D levels in both infants and their mothers. Therefore, physicians should monitor not only iron but also vitamin D levels in infants who are breastfed by mothers who had prepregnancy anemia.
There has been debate about the adequacy of the human milk for maintaining an ideal iron status and for providing other micronutrients in exclusively breastfed infants1). Given that maternal nutritional status affects the contents of breast milk, it can be hypothesized that the deficiency of iron and other nutrients in mothers may reduce the micronutrient stores in their infants.
It has been reported that infants born to anemic mothers have low iron stores and are more likely to develop anemia2,3). The American Academy of Pediatrics' (AAP) recommendations indicate that supplementation of oral iron drops before 6 months to breastfed infants may be needed to support iron stores4). However, several studies have shown that there is little correlation between maternal and neonatal ferritin concentrations5,6), and that maternal anemia does not affect breast milk iron or lactoferrin concentration at birth and during early lactation7).
Vitamin D stores in the fetus depend on maternal vitamin D status and breastfed infants continue to rely upon their mother's nutritional status. Previous studies have reported a high prevalence of Vitamin D deficiency (VDD) in breastfed infants8,9,10), and AAP recommended that all breastfed infants routinely should receive an oral supplement of vitamin D, 400 IU per day, beginning at hospital discharge to maintain an adequate serum vitamin D concentration11).
A concurrent deficit of micronutrients, including iron and vitamin D, in breastfed infants may result in a wide spectrum of adverse effects on growth, development and performance12). However, the iron and vitamin D status of breastfed infants in relation to the status of their mothers has not yet been sufficiently evaluated.
This retrospective study was performed to investigate the relationships between the iron and vitamin D status of breastfed infants and their mothers and to evaluate the risk factors for iron deficiency (ID) and VDD in breastfed infants.
Seventy breastfed infants aged 4 to 24 months and their mothers who visited Pediatric Department at the CHA Bundang Medical Center (Seongnam, Korea) were included in our study from February 2012 to May 2013. Exclusion criteria were (1) births at <35 weeks of gestation age or with low birth weight (≤2,500 g), (2) formula fed infants, and (3) mothers and infants who had taken micronutrient supplements including iron or vitamin D at enrollment.
Through the questionnaire, information about maternal history of pre- or postpregnancy anemia requiring iron supplementation, the pattern of menstruation after childbirth, maternal history of additional iron supplementation both during pregnancy and after delivery, the birth-related history, the duration of breast feeding and the weaning pattern were retrospectively checked. Additionally, laboratory test including the complete blood count, reticulocyte count, C-reactive protein (CRP), iron, total iron binding capacity, ferritin, calcium, phosphate, alkaline phosphatase, and 25-hydroxyvitamin D (25(OH)D) levels were performed both in infants and mothers. CRP was measured by high-sensitivity methods using an immunoturbidimetric assay. Serum ferritin was measured by chemiluminescence immunoassay. If the CRP level was greater than 0.3 mg/dL, we considered that serum ferritin had been affected by inflammation and as such excluded the ferritin data from statistical analysis (n=25). The serum 25(OH)D levels were measured by a radioimmunoassay kit from DiaSorin (Stillwater, MN, USA).
The infants were classified into iron deficiency anemia (IDA), ID, and normal groups. IDA was defined as hemoglobin (Hb)≤11 g/dL and ferritin≤12 ng/mL. IDA in infants with a CRP of more than 0.3 mg/dL was diagnosed as Hb≤11 g/dL with a mean corpuscular volume<70 fL and a transferrin saturation<16% (n=16). ID was defined as Hb>11 g/dL and ferritin≤12 ng/13). The maternal iron status was defined as ID if ferritin<50 ng/mL14). VDD was defined as 25(OH)D≤20 ng/mL, and vitamin D insufficiency (VDI) as 25(OH)D of 20-30 ng/mL15).
Data are expressed as means±standard deviations. The quantitative parameters of the groups were compared by t test. For comparing the 25(OH)D levels, we used analysis of variance to avoid interference by seasonal variation. Pearson correlation was performed to evaluate the relationship between the hematologic parameters and the 25(OH)D levels of infants and their mothers. Multivariable logistic regression was used to examine the influence of several variables on the iron and vitamin D status of breastfed infants. The PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. A P value < 0.05 and a confidence interval of 95% were considered statistically significant. This study was approved by the Institutional Review Board of the Medical Faculty at CHA University (BD 2014-0740).
The demographic and clinical characteristics of our study participants are shown in Table 1. The average birth weight was 3.2±0.4 kg. Eleven infants (16%) were born between 35 and 37 weeks of gestation, and no one before 35 weeks. The number of mothers with prepregnancy anemia was 21 (30%). The number of mothers who had taken additional iron supplements during pregnancy was 34 (49%).
Among 70 infants, 81% (n=57) had VDD. In terms of iron status, 60% (n=42) in infants had IDA, 10% (n=7) had ID, and 30% (n=21) had normal. Infants with IDA and VDD were 53% (n=37). Among mothers, 89% (n=62) had VDD and only 1% (n=1) had IDA.
Mothers with history of anemia before pregnancy had lower ferritin level than those without (P=0.034) (Table 2). The level of 25(OH)D was significantly lower in infants and their mothers with history of prepregnancy anemia than in those without. There was no difference in ferritin and 25(OH)D levels of mothers and infants according to the history of iron supplementation during pregnancy (data not shown).
The independent risk factors for ID or IDA in infants were body weight (P=0.04), and the duration of breast feeding (P=0.04) (Table 3).
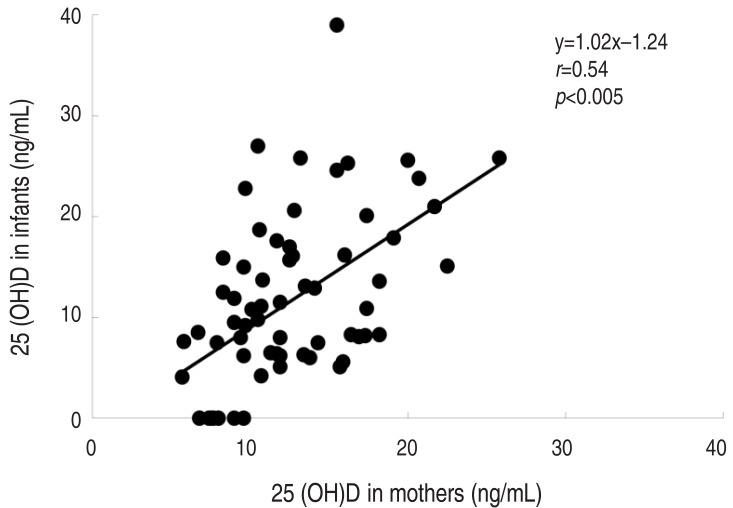
Our data show that a maternal prepregnancy history of anemia was associated with lower current ferritin and lower 25(OH)D in both infant and their mothers. The duration of breast feeding was the independent risk factors for ID. Maternal vitamin D levels significantly affected the level of vitamin D in their infants.
It was reported that deficiencies of vitamins, iron, and zinc occur concurrently in lactating mothers and their infants in rural villages in Indonesia16). In recent years, breast feeding has become more common in Korea. A recent epidemiologic survey in 2009 showed that the overall breast feeding rate in Korea was 89.0% and the exclusive BMF rate at 6 months of age was 49.3%17). The concurrent VDD and IDA in infants have also been observed in recent Korean studies18,19).
In this study, ferritin in mothers with prepregnancy anemia was significantly lower than in those mothers without a history of anemia. Neither maternal anemia nor prepregnancy history of anemia was related with ID in their infants. Several studies have shown that there is little correlation between maternal and neonatal ferritin concentration5,16). No correlation between maternal and neonatal ferritin level may come from the fact that breast milk iron concentration is usually consistent in various maternal iron status7). In lactating rats with different iron status, mammary gland transferrin receptors were inversely related to iron status20). It can be hypothesized that an anemic woman would have a large number of transferrin receptors in her mammary glands, to extract iron more efficiently from her serum.
More than half of breastfed infants had both VDD and IDA in the present study. In addition, the vitamin D of infants as well as mothers with prepregnancy anemia was lower than those without. Several possible mechanisms have been suggested to explain the association of VDD with anemia or ID in previous studies; ID decreased the levels of 1,25-dihydroxycholecalciferol in a rat model21). On the other hand, lower 25(OH)D levels were associated with an increased risk of anemia in a cross sectional study of 10,410 children and adolescents in the United States22). It was hypothesized that decreased calcitriol in bone marrow due to inadequate levels of 25(OH)D may limit erythropoiesis; calcitriol has a direct proliferative effect on erythroid burst-forming units that is synergistic with endogenously produced erythropoietin, and it also up-regulates expression of the erythropoietin receptor on erythroid progenitor cells23).
We found that lower birth weight or larger body weight increased the risk of ID. Previous studies have suggested that preterm or marginal low birth weight has a higher risk for ID, with smaller iron stores at birth and rapid growth24). An association between ID and overweight status has been observed in children25,26). Several factors have been proposed to explain this association, including genetic influences, alterations in iron metabolism, inadequate diet, and increased growth and body surface area27). We also found that the longer the duration of breastfeeding without supplement of iron-forified food, the greater ID in breastfed infants. Previous studies have suggested that breastfed infants rarely show IDA during the first six months as long as a sufficient amount of breast milk is provided, because the iron transferred through breast milk has high bioavailability28,29). The declining Hb and serum ferritin of infants beyond six months may be attributable to the normal depletion of iron stores not balanced by the exogenous supply of iron and the decline in the bioavailability of breast milk iron and the lactoferrin concentration30).
The close correlation between the vitamin D levels of infants and their mothers found in this study was also observed in previous research31). About 20% of maternal vitamin D is delivered through milk to the infant. A high prevalence of maternal VDD during lactation could contribute to VDD in exclusively breastfed infants32). In a study from Finland, it was found that an adequate supply of vitamin D in breast milk for breastfed infants could be accomplished only by increasing maternal vitamin D3 supplementation to 2,000 IU/day33). Recent studies have also shown that higher dose maternal vitamin D supplementation during lactation at 6,400 IU/day substantially increases vitamin D levels in mothers and infants without adverse effects34).
We found that the risk of vitamin D deficiency decreases with age in infants. It can be hypothesized that outdoor activities are increased with age, resulting in a positive effect on the cutaneous production of vitamin D.
The limitations of our study include its small number of subjects and the fact that this is a hospital-based study with possible selection bias. In addition, there was a lack of maternal medical record information including details of the amount of bleeding and the time of cord clamping at delivery as well as the socioeconomic data for our study group. There were also problems related to analyzing the ferritin level in infants who might have had inflammation without elevated CRP. Laboratory tests such as hepcidin, principal regulator of iron homeostasis to regulate iron transport across the gut mucosa, might be necessary in this situation35).
In conclusion, the maternal prepregnancy iron status and current maternal vitamin D has an effect on vitamin D of their breastfed infants. The prevalence of ID in infants significantly increased with the duration of breast feeding. Therefore, it is needed to concern about vitamin D as well as iron in breastfed infants with maternal prepregnancy anemia. In addition, a prospective research with a large number of subjects is needed to clarify micronutrient needs in pregnancy and during lactation for breastfed infants.
Conflicts of interest
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 2002;(1): CD003517

2. De Pee S, Bloem MW, Sari M, Kiess L, Yip R, Kosen S. The high prevalence of low hemoglobin concentration among Indonesian infants aged 3-5 months is related to maternal anemia. J Nutr 2002;132:2215–2221.


3. Morton RE, Nysenbaum A, Price K. Iron status in the first year of life. J Pediatr Gastroenterol Nutr 1988;7:707–712.


4. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841.


5. Rios E, Lipschitz DA, Cook JD, Smith NJ. Relationship of maternal and infant iron stores as assessed by determination of plasma ferritin. Pediatrics 1975;55:694–699.



6. Hussain MA, Gaafar TH, Laulicht M, Hoffebrand AV. Relation of maternal and cord blood serum ferritin. Arch Dis Child 1977;52:782–784.



7. Zavaleta N, Nombera J, Rojas R, Hambraeus L, Gislason J, Lönnerdal B. Iron and lactoferrin in milk of anemic mothers given iron supplements. Nutr Res 1995;15:681–690.

9. Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics 2006;118:603–610.


10. Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics 2014;133:e143–e153.


11. Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding. American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142–1152.


12. Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003;112:846–854.



13. Dube K, Schwartz J, Mueller MJ, Kalhoff H, Kersting M. Iron intake and iron status in breastfed infants during the first year of life. Clin Nutr 2010;29:773–778.


14. Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med 1992;7:145–153.


15. Huh SY, Gordon CM. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord 2008;9:161–170.


16. Dijkhuizen MA, Wieringa FT, West CE, Muherdiyantiningsih , Muhilal . Concurrent micronutrient deficiencies in lactating mothers and their infants in Indonesia. Am J Clin Nutr 2001;73:786–791.


17. Ministry of Health & Welfare. Korea Centers for Disease Control and Prevention. Korea Health Statistics 2009: Korea National Health and Nutrition Examination Survey (KNHANES IV-3). Cheongwon: Ministry of Health & Welfare, Korea Centers for Disease Control and Prevention, 2010.
18. Yoon JH, Park CS, Seo JY, Choi YS, Ahn YM. Clinical characteristics and prevalence of vitamin D insufficiency in children less than two years of age. Korean J Pediatr 2011;54:298–303.



19. Yoon JW, Kim SW, Yoo EG, Kim MK. Prevalence and risk factors for vitamin D deficiency in children with iron deficiency anemia. Korean J Pediatr 2012;55:206–211.



20. Sigman M, Lonnerdal B. Response of rat mammary gland transferrin receptors to maternal dietary iron during pregnancy and lactation. Am J Clin Nutr 1990;52:446–450.


21. Katsumata S, Katsumata-Tsuboi R, Uehara M, Suzuki K. Severe iron deficiency decreases both bone formation and bone resorption in rats. J Nutr 2009;139:238–243.


22. Atkinson MA, Melamed ML, Kumar J, Roy CN, Miller ER 3rd, Furth SL, et al. Vitamin D, race, and risk for anemia in children. J Pediatr 2014;164:153–158.e1.


23. Alon DB, Chaimovitz C, Dvilansky A, Lugassy G, Douvdevani A, Shany S, et al. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp Hematol 2002;30:403–409.


24. World Health Organization. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva: World Health Organization, 2001.
25. Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: risk factors and racial/ethnic disparities. Pediatrics 2007;120:568–575.


26. Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics 2004;114:104–108.


27. Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord 2003;27:416–418.


28. Raj S, Faridi M, Rusia U, Singh O. A prospective study of iron status in exclusively breastfed term infants up to 6 months of age. Int Breastfeed J 2008;3:3



29. Meinzen-Derr JK, Guerrero ML, Altaye M, Ortega-Gallegos H, Ruiz-Palacios GM, Morrow AL. Risk of infant anemia is associated with exclusive breast-feeding and maternal anemia in a Mexican cohort. J Nutr 2006;136:452–458.


30. Calvo EB, Galindo AC, Aspres NB. Iron status in exclusively breast-fed infants. Pediatrics 1992;90:375–379.



31. Saadi HF, Dawodu A, Afandi B, Zayed R, Benedict S, Nagelkerke N, et al. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Matern Child Nutr 2009;5:25–32.


32. Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr 2003;142:169–173.


33. Ala-Houhala M, Koskinen T, Parviainen MT, Visakorpi JK. 25-Hydroxyvitamin D and vitamin D in human milk: effects of supplementation and season. Am J Clin Nutr 1988;48:1057–1060.





 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


