Article Contents
| Clin Exp Pediatr > Volume 67(2); 2024 |
|
Abstract
Pneumonia is a common pediatric infectious disease that is familiar to pediatricians and a major cause of hospitalization worldwide. Recent well-designed epidemiologic studies in developed countries indicated that respiratory viruses are detected in 30%–70%, atypical bacteria in 7%–17%, and pyogenic bacteria in 2%–8% of children hospitalized with community-acquired pneumonia (CAP). The etiological distribution of CAP varies widely by child age and the epidemiological season of the respiratory pathogen. Moreover, diagnostic tests, particularly for the detection of Streptococcus pneumoniae and Mycoplasma pneumoniae, the 2 major bacterial pathogens involved in pediatric CAP, have several limitations. Therefore, management and empirical antimicrobial therapy for children with CAP should be applied in a stepwise manner based on recent epidemiological, etiological, and microbiological evidence.
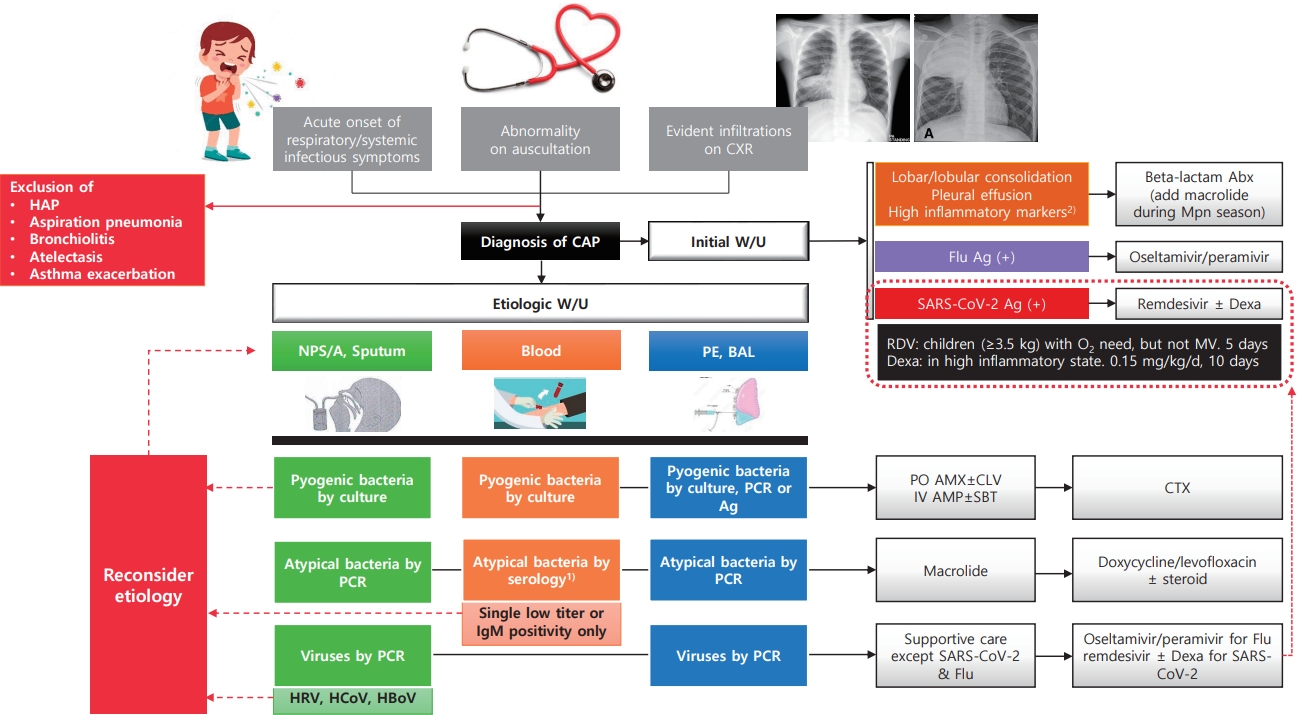
Graphical abstract. CCXR, chest radiograph; HAP, hospital-acquired pneumonia; CAP, community-acquired pneumonia; W/U, work-up; Abx, antibiotics; Mpn, Mycoplasma pneumoniae; Flu, influenza virus; Ag, antigen test; Dexa, dexamethasone; RDV, Remdesivir; MV, mechanical ventilation; NPS/A, nasopharyngeal swab/aspirate; PE, pleural effusion; BAL, bronchoalveolar lavage; PCR, polymerase chain reaction; HRV, human rhinovirus; HCoV, human coronavirus; HBoV, human bocavirus; PO, per oral; AMX, amoxicillin; CLV, clavulanate; IV, intravenous; AMP, ampicillin; SBT, sulbactam; CTX, ceftriaxone or cefotaxime. 1)4-fold increase in a paired serum sample or a single serologic test (IgG+IgM) titer of 1:640 or higher. 2)C-reactive protein or procalcitonin.
Pneumonia is a common infectious disease among children, familiar to pediatricians, and a major cause of hospitalization worldwide. Moreover, considering that most novel infectious diseases causing pandemics, including coronavirus disease 2019 (COVID-19), are caused by respiratory pathogens, the clinical significance of pneumonia is even greater. Despite its importance, particularly in children, the diagnosis, treatment, and prevention of pneumonia are based on studies conducted in adults. Thus, clinical studies have been conducted in developing and developed countries to improve the diagnosis and treatment of pneumonia in children. This review summarizes the results of major international and Korean studies conducted to date and proposes the most appropriate diagnosis and treatment policies accordingly.
Pneumonia is the leading cause of death in children aged <5 years worldwide. Estimated numbers of global deaths by pneumonia were 0.76 million, while the cause-specific mortality rate was 5.5 cases per 1,000 livebirths in 2015 [1]. Although mortality rates in developed countries do not reach the levels observed in low- and middle-income countries, the morbidity and financial burden associated with pneumonia remain significant. In a recent large epidemiological study in the United States (US), the annual incidence of community-acquired pneumonia (CAP) requiring hospitalization was 15.7 cases per 10,000 children, with the highest rate among children younger than 2 years [2].
Over the past 20 years, the incidence of lower respiratory tract infections (LRTIs) has declined worldwide. A total number of episodes of clinical pneumonia in young children (<5 years of age) in 132 developing countries decreased by 22% from 178×106 in 2000 to 138×106 in 2015 [3]. There has been a substantial decrease in the number of deaths and the mortality rate, which reflects not only economic development, improved nutrition, and reduced household crowding but also the use of pneumonia-specific interventions such as improved case management—including empirical antibiotic treatment—and effective vaccines against leading causes of pneumonia in children [4].
While the number of pneumonia cases and death rates have significantly decreased, hospital visits and hospitalization rates are increasing in both developing and developed countries, and the situation in South Korea is no exception [3,5]. According to the National Health Insurance Corporation database, the number of hospital visits increased from 9,509 to 12,833 per 100,000 population between 2004 and 2014, especially for those <10 years of age [5]. In an Asian multinational study, a total of 3,151 CAP patients <5 years of age were hospitalized in 2011 at 3 Korean hospitals, which accounted for 22.4% of all hospitalizations during that period. It was higher than Vietnam (5.4%), Malaysia (2.8%), and Indonesia (18.2%) [6]. In another study using National Emergency Department Information System data, a total of 329,380 children were diagnosed with pneumonia at Emergency Departments (EDs) nationwide between 2007 and 2014.
In the last 5 years (March 2015 to February 2020) before the COVID-19 pandemic, the number of CAP cases in children in 4 referring hospitals in the Korean metropolitan area was 1.0/100 inpatients in the Department of Pediatrics. It showed the highest incidence in the winter season, when respiratory syncytial virus (RSV) and Mycoplasma pneumoniae were prevalent, and another increasing trend in March and September with the opening of schools. However, the COVID-19 pandemic had a significant impact on the epidemiology of other respiratory viruses, showing a different epidemiology of pneumonia from that noted before the pandemic [7]. Nevertheless, the incidence of CAP increased significantly due to the circulation of RSV-B in the winter season of 2021 and human metapneumovirus and RSV-Ain the fall and winter of 2022 (unpublished data).
As mentioned above, the use of effective vaccines such as pneuococcal conjugate vaccine (PCV), Haemophilus influenza type b vaccine, and influenza vaccine, improved hygiene and living environment, and the efforts of the World Health Organization (WHO) and each country to reduce pneumonia have greatly reduced global morbidity and mortality rates [3,4]. At the same time, changes in the distribution of the causative pathogens of pneumonia are expected. To enable the appropriate diagnosis, treatment, and prevention of pneumonia, it is important to understand the distribution of the causative pathogens. Large-scale epidemiological and etiological studies have recently been conducted to address this urgent and unmet need.
Efforts to enhance our understanding of the etiology of pneumonia have been initiated in developing countries. The Drakenstein study was conducted in 2012–2014 by the Bill and Melinda Gates Foundation using the South African birth cohort [8]. Two large-scale multicenter prospective case-control cohort studies of pneumonia etiology in children have been conducted: the GABRIEL (Global Approach to Biological Research, Infectious disease, and Epidemics in Low-income countries) in 2010–2014 [9] and the PERCH (Pneumonia Etiology Research for Child Health) study in 2011–2014 [10]. These 2 studies involved infants and toddlers <5 years of age who were hospitalized for pneumonia (Table 1).
Among developed countries, the US first conducted a multicenter prospective case-control study for pneumonia etiology in children during 2010–2012, the well-known EPIC (Etiology of Pneumonia in the Community) study [2]. A total of 2,638 children with CAP requiring hospitalization were enrolled in 3 hospitals over 2.5 years. In this study, 89% of patients had radiographic evidence of pneumonia, and pyogenic bacterial pathogens were detected only in the blood, pleural effusion, and bronchoalveolar lavage (BAL) fluid using both polymerase chain reaction (PCR) and conventional culture techniques. As a result of this comprehensive etiologic work-up for CAP in children, pathogens were detected in 81%, viruses in 73%, pyogenic bacteria in 7%, and atypical bacteria such as M. pneumoniae in 8%. Similarly, another US study was conducted to get data on the clinical characteristics and etiology of CAP in both inpatients and outpatients during 2015–2018. In this study, except for S. pneumoniae, there were no significant differences between inpatients and outpatients in the proportions of children with specific pathogens detected [11]. The main strengths of these studies include the prospective collection of standardized data using advanced molecular diagnostic techniques.
To date, well-designed comprehensive etiological studies of CAP in children have rarely been conducted in Asia. A Japanese study in 2005–2006 investigated the detection frequency of pathogens in nasopharyngeal swabs of children with CAP using real-time PCR and bacterial culture. S. pneumoniae and M. pneumoniae were the most commonly detected at 24% and 15%, respectively. Among viruses, rhinovirus was detected most commonly at 14.5% [12]. This result showed that S. pneumoniae colonized the nasopharynx of children before the introduction of PCV in 2010. However, in a Taiwanese study conducted in 2010–2013 at 8 participating medical centers, the prevalence of S. pneumoniae was very high despite the introduction of PCV in 2005.In this study, respiratory specimens were excluded from the detection of S. pneumoniae; however, they incorporated urinary antigen test results, which could not differentiate between colonization and true infection, particularly in children. The most common pathogen was S. pneumoniae (31.6%), but it was detected using only the urinary antigen test (83.1%). In addition, they mainly used serological tests, which have relatively low specificity, to detect M. pneumoniae, the second most commonly identified pathogen (22.6%) [13].
In China, a multicenter prospective study was conducted on the etiology of radiologically confirmed CAP in hospitalized children aged 6 months to 14 years in 2015. They tested only 8 respiratory viruses from oropharyngeal swabs using the direct fluorescent antibody technique, used an immunoglobulin M (IgM) serologic test for M. pneumoniae detection, and did not report the test results for pyogenic bacteria. M. pneumoniae was the most frequently detected pathogen (32.4%) [14]. In the major Asian studies above, the rates of pyogenic bacteria and M. pneumoniae were reportedly very high, including culture and/or PCR results from upper respiratory tract specimens and serological tests, respectively.
In South Korea, there have been no systematic analyses of the etiology of pneumonia in children. However, the detection rates of the respiratory viruses for acute LRTI were studied by several researchers between 2000 and 2011, varying from 10% to 73% (Table 2) [15-20]. The most commonly detected viruses were RSV and parainfluenza virus, although adenovirus and human rhinovirus (HRV) were the second most commonly detected viruses in some studies [17,20]. These studies are very limited because bronchiolitis was included and bacterial pathogens were not investigated. On the other hand, in a study of 122 cases of empyema diagnosed in 35 hospitals nationwide in 1999–2004, S. pneumoniae was the most common (36.9%), followed by Streptococcus pyogenes (6.6%) and Staphylococcus aureus (5.7%) [21]. Moreover, in the analysis of 288 cases of lobar/ lobular pneumonia diagnosed at a single institution in 2006–2008, pyogenic bacteria, which mostly consisted of S. pneumoniae (88.9%), were identified in 5.9% of blood and respiratory cultures versus M. pneumoniae in 50.7% of serologic tests [22].
A large-scale multicenter study of CAP etiology was recently conducted by a pediatric research network [23]. This was a retrospective study of 30,994 children with CAP hospitalized in 2010–2015 at 23 hospitals. This study did not include data on pyogenic bacteria. The same group recently conducted a multicenter prospective study of CAP etiology in 1,023 children in 2018–2020 at 28 hospitals nationwide [24]. M. pneumoniae (41.3%) and viruses (65.7%) with HRV (30.5%) were commonly detected. A total of 264 bacterial strains (25.8%), isolated by culture and/or PCR: S. aureus (13%), S. pneumoniae (9%), and H. influenzae (2%). The proportions of M. pneumoniae and pyogenic bacteria in this study were significantly higher than those reported in other Asian studies. These findings may indicate that colonized pyogenic bacteria might be misidentified as a pathogen and that the result of the serologic test with low specificity for M. pneumoniae was mainly used for the etiologic diagnosis.
To evaluate the etiologic distribution of CAP in Korean children from a different perspective, a retrospective multicenter study was recently conducted by another group [7]. In this study, a rigorous case definition for CAP was applied with evident clinical symptoms, physical examination, and chest radiographic findings corresponding to pneumonia. In addition, upper respiratory tract specimens were excluded to detect pyogenic bacteria and colonization. Serological test results for M. pneumoniae were considered positive only when a 4-fold increase between paired blood samples or a much higher (usually 10 times) cutoff value with a single antibody titer was identified. In addition, HRV, human bocavirus (HBoV), and human coronavirus (HCoV) were excluded as pathogens because they are often detected in the nasopharynx of asymptomatic children and their etiologic role in CAP is not evident in children. In the last 5 years (March 2015 to February 2020) before the COVID-19 pandemic, respiratory viruses were detected in 31.6%, atypical bacteria in 17.4%, and pyogenic bacteria in only 2.3% of children hospitalized with CAP. No pathogens were detected in approximately half of the children. The most common pathogens were M. pneumoniae (16.8%) and RSV (13.7%), and the most common pyogenic bacterial species were S. pneumoniae (0.6%) and S. pyogenes (0.6%).
The diagnosis of pneumonia is based on respiratory symptoms, physical examination, and/or chest radiographic findings. However, in the absence of clear alveolar consolidation and PE on chest radiography (CXR), it may be difficult to distinguish other LRTI such as croup, bronchitis, and bronchiolitis from pneumonia. The case definitions for pneumonia of the WHO are mainly used in developing countries, and the severity is classified by the degree of respiratory distress. Pneumonia is defined as cough/difficulty breathing and age-adjusted tachypnea [25]. In addition, the etiological diagnosis of pneumonia is complicated and difficult in typical clinical situations.
In particular, bacterial pneumonia, such as that caused by S. pneumoniae, the most important pathogen in CAP because it should be treated with antibiotics, is difficult to accurately identify in children [26]. A small proportion of cases are detected by blood and/or PE culture, even under ideal conditions. Sputum cannot be adequately produced by young children but can be easily contaminated by organisms present in the nasopharynx. In addition, the detection of pneumococcus in the nasopharynx or positivity on a urinary antigen test may indicate asymptomatic carriage, which is prevalent even in healthy young children. When pneumococci are present in the nasopharynx, genomic fragments of the bacteria can be detected in the blood through PCR. Therefore, blood PCR for pneumococcus has low specificity in children with CAP [26]. Moreover, diagnostic serology is insensitive in children, and paired samples are difficult to obtain. BAL or lung biopsy, performed to avoid contamination with upper respiratory secretions, is the gold standard diagnostic technique for pneumonia; however, these procedures are rarely performed in children with CAP because of their invasiveness. Thus, even with a sufficient etiologic work-up, the diagnosis of some bacterial pneumonias may be missed, even in cases of pneumonia for which the causative bacteria have not been identified or respiratory viruses have been detected.
Therefore, it is common to use empirical antibiotics based on clinical findings suggesting the possibility of pyogenic bacterial pneumonia, such as lobar/lobular consolidation, empyema/PE, and high inflammatory markers (C-reactive protein and procalcitonin). In particular, if these findings occur outside the M. pneumoniae epidemic period (which may have similar clinical manifestations) or in children who have not completed PCV immunization, the probability of a pneumococcal etiology of this pneumonia might be high. Recent studies additionally suggested that the nasopharyngeal carriage load of pneumococcus is higher in pneumococcal CAP than in other etiologic CAP and thus can be used to diagnose pneumococcal CAP [27-29].
Atypical pneumonia pathogens include M. pneumoniae, Chlamydophila pneumoniae, Chlamydophila trachomatis, and Legionella pneumophilia; however, M. pneumoniae accounts for most of the causes of atypical CAP in children. However, in neonates and infants less than 3 months of age, C. trachomatis can manifest similar to viral pneumonia such as RSV. A repetitive staccato cough, tachypnea, and rales are characteristic, but wheezing is uncommon [30]. All atypical bacteria are usually detected by PCR in respiratory samples because culturing is difficult and time-consuming. Recently, multiplex PCR kits containing major respiratory viruses and atypical bacteria have been commercialized and are now frequently used in clinical settings [31].
However, caution is required for interpretation as M. pneumoniae may be detected in nasopharyngeal swabs from asymptomatic healthy children depending on the time of prevalence, region, target age, and race [32]. Mycoplasma PCR in sputum samples show widely distributed sensitivity of 9%–100%, has poor concordance with paired serology, and does not reliably differentiate infection from colonization [33]. On the other hand, serology is among the most widely used methods for the diagnosis of M. pneumoniae. A four-fold or higher increase in antibody levels in acuteconvalescent serum is considered diagnostic, but it is difficult to apply in clinical settings. Therefore, a single IgM titer is usually measured, but it can remain high for months or possibly years, may not appear in very young children or during reinfection, and the positive predictive value can be as low as 15% [32-34]. However, a single antibody titer of 1:640 or higher in an antibody test including both immunoglobulin G (IgG) and IgM was highly concordant with the positive results of a Mycoplasma PCR test from nasopharyngeal aspirates in Korean children [35]. Thus, we may use a high single titer of serologic (IgG + IgM) test for M. pneumoniae to enhance its specificity, although the sensitivity is probably reduced, particularly in school-aged children and adolescents with CAP during the M. pneumoniae epidemic.
As mentioned above, atypical bacterial pneumonia, particularly M. pneumoniae pneumonia, has limitations in clinical diagnosis because it can present with various symptoms, severities, and laboratory and CXR findings. However, if a school-aged child who has received all PCV doses develops CAP accompanied by lobar/lobular consolidation or PE during an M. pneumoniae epidemic, the possibility of M. pneumoniae pneumonia may be high [36,37].
The detection of respiratory viruses in patients with pneumonia is mainly performed on nasopharyngeal aspirate or swab samples using commercially available multiplex real-time reverse transcription PCR (RT-PCR) Unlike most bacteria, respiratory viruses have a low probability of asymptomatic colonization; therefore, those detected in children and adolescents with pneumonia are generally accepted as causative agents. However, HRV, HBoV, and HCoV are the exceptions. Because these viruses often cause asymptomatic infection or can shed for a long time after infection, they are detected at a similar rate in the asymptomatic control group as in patients with pneumonia [2,10,11,38]. Serology can support RT-PCR, but it is also clinically limited because of possible false-positive and -negative results; thus, acute and convalescent serum should be obtained [39].
Syndromic multiplex PCR panels have been developed for this purpose. It enables the detection of viruses, atypical bacteria, pyogenic bacteria, and antimicrobial resistance marker genes in respiratory specimens. Semiquantitative results were also obtained for bacterial targets. The panel showed a sensitivity of 100% and specificity of 87.2% [31]. Metagenomics and pan-viral group PCR can detect additional viruses, some of which are known to be pathogenic, in nasopharyngeal/oropharyngeal specimens from one-third of children hospitalized with CAP of unknown etiology. Both broad-range methods could be useful tools in future epidemiological and diagnostic studies [40]. Cell-free plasma next-generation sequencing was made available in 2017 to supplement the standard of care diagnostic techniques. In a previous study in the US, among 15 children hospitalized with CAP, a pathogen was identified in 13 of 15 children (86 %) with cell-free plasma sequencing compared with 47% for those using standard culture and PCR-based methods alone [41]. RNA sequencing profiles by transcriptional analysis of blood from infants with RSV LRTI allow specific diagnosis, better understanding of disease pathogenesis, and assessment of disease severity. This technique may open a new era for the identification of potential therapeutic or preventive targets, if applied in an appropriate clinical setting [42].
Among children with CAP in developed countries, 60%–90% might have a viral etiology; thus, they would require conservative management with symptomatic care during the disease course. However, children with presumed bacterial and influenza virus pneumonia require empirical antimicrobial therapy during their initial presentations. The Pediatric Infectious Disease Society and Infectious Disease Society of America (IDSA) in the US recommended amoxicillin or ampicillin for children with presumed pyogenic bacterial pneumonia, azithromycin for children with presumed atypical pneumonia, and oseltamivir or zanamivir for children with presumed influenza pneumonia [43]. The Korea Centers for Disease Control and Prevention (KCDC) similarly recommended the 2017 guidelines for antibiotic use in children with LRTI [44].
However, clinical suspicion of the etiology of CAP in children is difficult and inaccurate; therefore, most clinicians usually prescribe antibiotics. Thus, unless pyogenic bacterial pneumonia is carefully considered, the effect of empirical amoxicillin administration is generally insignificant. This has been evidenced by several well-designed large-scale randomized clinical trials in developing countries. In Malawi children aged 2–59 months, placebo treatment for nonsevere fast-breathing pneumonia was significantly inferior to amoxicillin treatment in terms of overall treatment failure. However, by day 4, approximately 93% of children receiving placebo did not experience treatment failure, and there was no significant difference between groups in treatment failure or relapse by day 14 [45]. In Pakistan, among children younger than 5 years of age with nonsevere pneumonia, the frequency of treatment failure was higher in the placebo than amoxicillin group, but a difference that did not meet the noninferiority margin for placebo [46]. Also, in the US, among children who visited the ED with suspected CAP, treatment failure or admission within 30 days was not statistically different between those who did and did not receive an antibiotic prescription [47].
In terms of antibiotic treatment duration, 10-day treatment courses of amoxicillin have been best studied and recommended by both 2011 IDSA and 2017 KCDC guidelines [43,44]. However, a 5-day course of high-dose oral amoxicillin was not inferior to a 10-day course in 6- to 59-month-old outpatients with alveolar CAP, which is more likely to have a pyogenic bacterial cause.48) In Malawian children, 3-day treatment with amoxicillin for chest-indrawing pneumonia was noninferior to 5-day treatment [49]. In the United Kingdom, among children with CAP discharged from an ED or hospital ward (within 48 hours), lower-dose outpatient oral amoxicillin was noninferior to higher-dose amoxicillin and 3-day duration was noninferior to 7 days in terms of the need for antibiotic re-treatment [50]. In the SAFER (Short-Course Antimicrobial Therapy for Pediatric Respiratory Infections) trial in Canada, short-course (5-day) antibiotic therapy appeared comparable to standard care (10-day) for the treatment of previously healthy children with CAP not requiring hospitalization [51].
Macrolides are recommended as first-line therapy; however, macrolide resistance rates to M. pneumoniae among children have been increasing substantially. Macrolide resistance does not contribute to the clinical severity of M. pneumoniae pneumonia; however, it may be an aggravating factor. Antibiotics may not be required for treatment in mild cases due to the self-resolving nature of M. pneumonia infection regardless of macrolide resistance [52]. The clinical benefit of tetracyclines and fluoroquinolones has been shown in terms of shortening symptom duration and achieving rapid defervescence in some reports. However, due to safety concerns regarding these 2 alternative antibiotics, clinicians should weigh the risks and benefits when selecting treatment options. Alternative antibiotics may be considered when patients remain febrile or when chest x-rays show deterioration at least 48–72 hours after macrolide treatment [53]. The detailed recommendations for the treatment of macrolide-resistant M. pneumoniae pneumonia can be found in the 2019 guideline codeveloped by the Korean Society of Pediatric Infectious Disease and the Korean Academy of Pediatric Allergy and Respiratory Disease [54] and would not be covered here as it is beyond the scope of this review.
Among the viral pathogens that cause CAP in children, influenza virus and severe acute respiratory syndrome coronavirus 2 are currently recommended treatment with antiviral agents. Early treatment with oseltamivir reduces the illness and hospitalization durations for patients with serious illness or those with ongoing clinical deterioration [55,56]. Zanamivir (in ≥7 years-old) and peramivir (in ≥6 months-old) could also be administered by inhalation and parenterally, respectively, for the treatment of influenza pneumonia [57]. Remdesivir is suggested for children (≥3.5 kg) with severe COVID-19 including pneumonia who need supplemental oxygen without mechanical ventilation. Nirmatrelvir/ritonavir is considered for adolescents (≥12 years and ≥40 kg) at high risk of progression to severe disease who do not require supplemental oxygen and are within 5 days of symptom onset [58]. High risk factors include obesity, diabetes, heart disease, chronic lung diseases, seizure disorders, and an immunocompromised status [59]. Otherwise, for the treatment of other common respiratory viruses causing CAP in children, specific antiviral therapies including ribavirin for RSV and cidofovir for adenovirus are not usually recommended [43].
Clinical trials have yielded conflicting data regarding the benefits of adding systemic corticosteroids to CAP treatment. Recent randomized clinical trials conducted in adults indicated that short-term corticosteroid treatment reduces the time to clinical stability in patients admitted to the hospital for CAP [60] or reduces the risk of treatment failure among patients with severe CAP and a high initial inflammatory response [61], but these effects were not clinically significant. Pediatric studies must evaluate the strengths and weaknesses of steroid therapy in children with CAP. However, for some pathogens, the effectiveness of corticosteroids has been relatively well evaluated and corticosteroids are recommended for treatment in some situations. First, early corticosteroid therapy reduces disease morbidity in children with CAP caused by M. pneumoniae, particularly macrolide-resistant M. pneumoniae, without increasing the incidence of adverse reactions [62,63]. In addition, corticosteroids are recommended for children and adolescents with severe to critical COVID-19. This might reduce excessive immune and inflammatory responses during the severe course of COVID-19 pneumonia [58].
Regarding the recent epidemiology and diagnostic and therapeutic advances, we recommend a management strategy for CAP in otherwise healthy children (Fig. 1).
CAP is a common infectious disease that can be easily diagnosed and treated by all pediatricians. However, it results in unnecessary medical resource waste, excessive antibiotic administration, and hospitalization because an accurate diagnosis and appropriate treatment are frequently lacking. As evidence-based scientific diagnosis and treatment policies have become the basis for overcoming emerging infectious diseases such as COVID-19, multidisciplinary clinical studies are needed to better understand and appropriately diagnose, treat, and prevent CAP in children.
Fig. 1.
CXR, chest x-ray; PE, pleural effusion; Flu, influenza; Mpn, Mycoplasma pneumoniae; LFX, levofloxacin; DOX, doxycycline; COVID-19, coronavirus disease 2019. a)Lobar/lobular consolidation, not fully vaccinated with pneumococcal conjugate vaccine, or high concentration of inflammatory markers (C-reactive protein >10 mg/dL and/or procalcitonin >5 mg/dL) during the no Mpn epidemic season.
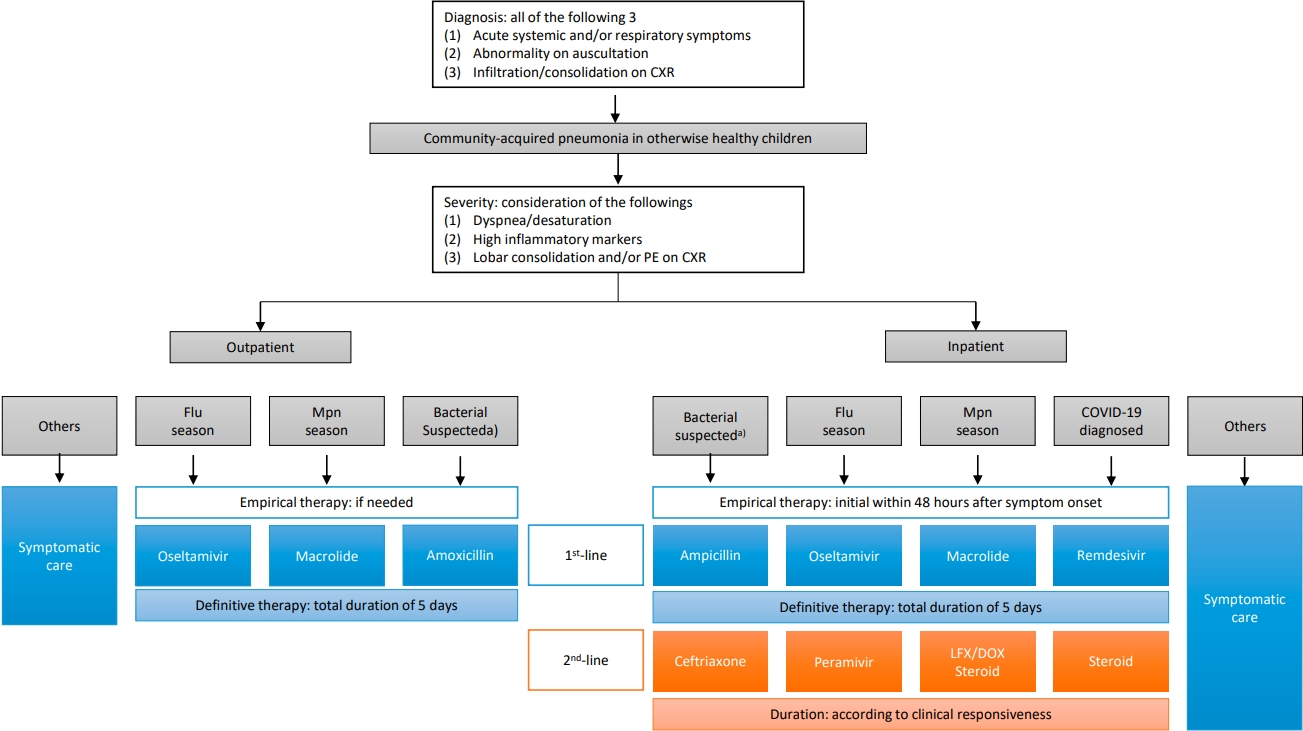
Table 1.
Summary of international studies of etiology of CAP in children
| Variable | GABRIEL [9] | PERCH [10] | EPIC [2] | CHIRP [11] |
|---|---|---|---|---|
| Region/center | Multinational (n=8)a) | Multinational (n=7)b) | US (3 hospitals) | US (6 hospitals) |
| Period | 2010–2014 | 2011–2014 | 2010–2012 | 2015–2018 |
| Setting | Prospective observational case-control | Prospective observational case-control | Prospective observational case-control | Prospective observational case-control |
| Subject | Hospitalized | Hospitalized | Hospitalized | Hospitalized and outpatient clinic |
| Age | 2–60 Months | 1–59 Months | <18 Years | 2 Months–18 years |
| CAP category included | Radiologically confirmed, primary end-point pneumonia (WHO [64])c) | WHO-defined severe or very severe pneumonia [65] with a positive x-ray | Radiologically confirmed | Radiologically confirmed |
| Cases (n) | 888 | 1,769 | 2,222 | 441 |
| Controls (n) | 870 | 5,102 | 521 | 50 |
| Pathogen detected | NA | 98.2% | 81% | 64.6% |
| Virus | 78.3% | 61.4% | 73% | 55.6% |
| HRV (24.9%), RSV (20.0%), bocavirus (9.2%) | RSV (31.1%), HRV (NA), HMPV (NA) | RSV (28%), HRV (27%), HMPV (13%) | HRV/EV (18.6%), RSV (16.8%), HMPV (10.0%) | |
| Pyogenic bacteria | 15.2% | 27.3% | 7% | 4.3% |
| Spn (9.9), Hib (2.7), SA (2.0) | NA | Spn (4%) | Spn (2.3%) | |
| Atypical bacteria | 1.9% | NA | 8% | 8.80% |
| Mpn (1.5%), Cpn (0.4%) | NA | Mpn (8%) | Mpn (8.2%) |
CAP, community-acquired pneumonia; GABRIEL, Global Approach to Biological Research, Infectious disease, and Epidemics in Low-income countries; PERCH, Pneumonia Etiology Research for Child Health; EPIC, Etiology of Pneumonia in the Community; CHIRP, Conventional Versus Hypofractionated Radiation in High Risk Prostate Patients; US, United States; WHO, World Health Organization; HRV, human rhinovirus; RSV, respiratory syncytial virus; HMPV, human metapneumovirus; NA, not available; Spn, Streptococcus pneumoniae; Hib, Haemophilus influenza type b; SA, Staphylococcus aureus; Mpn, Mycoplasma pneumoniae; Cpn, Chlamydia pneumoniae.
Table 2.
Proportion of viral etiologies among acute lower respiratory tract infections in Korean children in previous studies
References
1. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388:3027-35.



2. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372:835-45.


3. McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2019;7:e47-57.


4. GBD 2017 Lower Respiratory Infections Collaborators. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: an analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2020;20:60-79.


5. Jeon YH, Kim JH. Treatment of community-acquired pneumonia in Korean children. Allergy Asthma Respir Dis 2017;5:177-84.


6. Tan KK, Dang DA, Kim KH, Kartasasmita C, Kim HM, Zhang XH, et al. Burden of hospitalized childhood community-acquired pneumonia: a retrospective cross-sectional study in Vietnam, Malaysia, Indonesia and the Republic of Korea. Hum Vaccin Immunother 2018;14:95-105.



7. Suh JH, Yun KW, Han MS, Choi SJ, Lee H, Park JY, et al. Etiology and clinical characteristics of community-acquired pneumonia in Korean children during pre-COVID-19 pandemic period, 2015-2020. In: Presentation at 71th Korean Academy of Pediatrics Annual meeting; 2022 Oct. Seoul, Korea.
8. Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med 2016;4:463-72.



9. Benet T, Sanchez Picot V, Messaoudi M, Chou M, Eap T, Wang J, et al. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: the GABRIEL pneumonia multicenter, prospective, case-control study. Clin Infect Dis 2017;65:604-12.

10. Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019;394:757-79.


11. Yun KW, Wallihan R, Desai A, Alter S, Ambroggio L, Cohen DM, et al. Clinical characteristics and etiology of community-acquired pneumonia in US children, 2015-2018. Pediatr Infect Dis J 2022;41:381-7.


12. Hamano-Hasegawa K, Morozumi M, Nakayama E, Chiba N, Murayama SY, Takayanagi R, et al. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother 2008;14:424-32.



13. Chi H, Huang YC, Liu CC, Chang KY, Huang YC, Lin HC, et al. Characteristics and etiology of hospitalized pediatric community-acquired pneumonia in Taiwan. J Formos Med Assoc 2020;119:1490-9.



14. Oumei H, Xuefeng W, Jianping L, Kunling S, Rong M, Zhenze C, et al. Etiology of community-acquired pneumonia in 1500 hospitalized children. J Med Virol 2018;90:421-8.




15. Moon JH, Suh KJ, Chung EH, Shin MY, Lee JS, Park YM, et al. Epidemiology of acute viral llower respiratory tract infection in hospitalized children in two different areas of Korea. Korean J Pediatr Infect Dis 2002;9:193-200.


16. Kwon JH, Chung YH, Lee NY, Chung EH, Ahn KM, Lee SI. An epidemiological study of acute viral lower respiratory tract infections in hospitalized children from 2002 to 2006 in Seoul, Korea. Pediatr Allergy Respir Dis 2008;18:26-36.
17. Cheong HY, Lee JH, Kim YB, Nam HS, Choi YJ, Kim CJ, et al. Viral etiologic agents in acute viral lower respiratory tract detected by multiplex RT-PCR. Pediatr Allergy Respir Dis 2007;17:334-53.
18. Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis 2006;43:585-92.



19. Kim KH, Lee JH, Sun DS, Kim YB, Choi YJ, Park JS, et al. Detection and clinical manifestations of twelve respiratory viruses in hospitalized children with acute lower respiratory tract infections : focus on human metapneumovirus, human rhinovirus and human coronavirus. Korean J Pediatr 2008;51:834-41.

20. Kim HY, Kim KM, Kim SH, Son SK, Park HJ. Clinical manifestations of respiratory viruses in hospitalized children with acute viral lower respiratory tract infections from 2010 to 2011 in Busan and Gyeongsangnam-do, Korea. Pediatr Allergy Respir Dis 2012;22:265-72.

21. Yum HY, Kim WK, Kim JT, Kim HH, Rha YH, Park YM, et al. The causative organisms of pediatric empyema in Korea. Korean J Pediatr 2007;50:33-9.

22. Lee YH, Shin YL, Suh WS, Shin MY, Park JO. A clinical study of lobar/lobular pneumonia in children. Pediatr Allergy Respir Dis 2009;19:271-81.
23. Lee E, Kim CH, Lee YJ, Kim HB, Kim BS, Kim HY, et al. Annual and seasonal patterns in etiologies of pediatric community-acquired pneumonia due to respiratory viruses and Mycoplasma pneumoniae requiring hospitalization in South Korea. BMC Infect Dis 2020;20:132.




24. Roh EJ, Lee MH, Lee JY, Kim HB, Ahn YM, Kim JK, et al. Analysis of national surveillance of respiratory pathogens for community-acquired pneumonia in children and adolescents. BMC Infect Dis 2022;22:330.


25. World Health Organization. Revised WHO classification and treatment of pneumonia in children at health facilities: Evidence summaries [Internet]. Geneva (Switzerland): World Health Organization; 2014 [cited Year Month Day]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK264164/.
26. Morpeth SC, Deloria Knoll M, Scott JAG, Park DE, Watson NL, Baggett HC, et al. Detection of pneumococcal dna in blood by polymerase chain reaction for diagnosing pneumococcal pneumonia in young children from low- and middle-income countries. Clin Infect Dis 2017;64(suppl_3): S347-56.



27. Albrich WC, Madhi SA, Adrian PV, van Niekerk N, Mareletsi T, Cutland C, et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis 2012;54:601-9.



28. Baggett HC, Watson NL, Deloria Knoll M, Brooks WA, Feikin DR, Hammitt LL, et al. Density of upper respiratory colonization with Streptococcus pneumoniae and its role in the diagnosis of pneumococcal pneumonia among children aged <5 years in the PERCH study. Clin Infect Dis 2017;64(suppl 3): S317-27.


29. Esposito S, Zampiero A, Terranova L, Ierardi V, Ascolese B, Daleno C, et al. Pneumococcal bacterial load colonization as a marker of mixed infection in children with alveolar community-acquired pneumonia and respiratory syncytial virus or rhinovirus infection. Pediatr Infect Dis J 2013;32:1199-204.


30. Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis 2005;16:235-44.


31. Murphy CN, Fowler R, Balada-Llasat JM, Carroll A, Stone H, Akerele O, et al. Multicenter evaluation of the biofire filmarray pneumonia/pneumonia plus panel for detection and quantification of agents of lower respiratory tract infection. J Clin Microbiol 2020;58:e00128-20.




32. Spuesens EB, Fraaij PL, Visser EG, Hoogenboezem T, Hop WC, van Adrichem LN, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 2013;10:e1001444.



33. Copete AR, Vera C, Herrera M, Aguilar YA, Rueda ZV, Velez LA. Mycoplasma pneumoniae in children with and without community-acquired pneumonia. What do PCR and serology say? Pediatr Infect Dis J 2020;39:e104-8.


34. Lee WJ, Huang EY, Tsai CM, Kuo KC, Huang YC, Hsieh KS, et al. Role of serum Mycoplasma pneumoniae IgA, IgM, and IgG in the diagnosis of Mycoplasma pneumoniae-related pneumonia in school-age children and adolescents. Clin Vaccine Immunol 2017;24:e00471-16.




35. Kim NH, Lee JA, Eun BW, Shin SH, Chung EH, Park KW, et al. Comparison of polymerase chain reaction and the indirect particle agglutination antibody test for the diagnosis of Mycoplasma pneumoniae pneumonia in children during two outbreaks. Pediatr Infect Dis J 2007;26:897-903.


36. Gordon O, Oster Y, Michael-Gayego A, Marans RS, Averbuch D, Engelhard D, et al. The clinical presentation of pediatric Mycoplasma pneumoniae infections - a single center cohort. Pediatr Infect Dis J 2019;38:698-705.


37. Kutty PK, Jain S, Taylor TH, Bramley AM, Diaz MH, Ampofo K, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis 2019;68:5-12.



38. Self WH, Williams DJ, Zhu Y, Ampofo K, Pavia AT, Chappell JD, et al. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016;213:584-91.


39. Zhang Y, Sakthivel SK, Bramley A, Jain S, Haynes A, Chappell JD, et al. Serology enhances molecular diagnosis of respiratory virus infections other than influenza in children and adults hospitalized with community-acquired pneumonia. J Clin Microbiol 2017;55:79-89.




40. Schlaberg R, Queen K, Simmon K, Tardif K, Stockmann C, Flygare S, et al. Viral pathogen detection by metagenomics and pan-viral group polymerase chain reaction in children with pneumonia lacking identifiable etiology. J Infect Dis 2017;215:1407-15.



41. Farnaes L, Wilke J, Ryan Loker K, Bradley JS, Cannavino CR, Hong DK, et al. Community-acquired pneumonia in children: cell-free plasma sequencing for diagnosis and management. Diagn Microbiol Infect Dis 2019;94:188-91.



42. Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 2013;10:e1001549.



43. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53:e25. -76.




44. Korean Disease Control and Prevention. Guidelines for the antibiotic use in children with lower respiratory tract infections. Cheongju (Korea): Korean Disease Control and Prevention,, 2017.
45. Ginsburg AS, Mvalo T, Nkwopara E, McCollum ED, Ndamala CB, Schmicker R, et al. Placebo vs amoxicillin for nonsevere fast-breathing pneumonia in Malawian children aged 2 to 59 months: a double-blind, randomized clinical noninferiority trial. JAMA Pediatr 2019;173:21-8.



46. Jehan F, Nisar I, Kerai S, Balouch B, Brown N, Rahman N, et al. Randomized trial of amoxicillin for pneumonia in Pakistan. N Engl J Med 2020;383:24-34.



47. Lipshaw MJ, Eckerle M, Florin TA, Crotty EJ, Lipscomb J, Jacobs J, et al. Antibiotic use and outcomes in children in the emergency department with suspected pneumonia. Pediatrics 2020;145:e20193138.




48. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J 2014;33:136-42.

49. Ginsburg AS, Mvalo T, Nkwopara E, McCollum ED, Phiri M, Schmicker R, et al. Amoxicillin for 3 or 5 days for chest-indrawing pneumonia in Malawian children. N Engl J Med 2020;383:13-23.



50. Bielicki JA, Stohr W, Barratt S, Dunn D, Naufal N, Roland D, et al. Effect of amoxicillin dose and treatment duration on the need for antibiotic re-treatment in children with community-acquired pneumonia: the CAP-IT randomized clinical trial. JAMA 2021;326:1713-24.


51. Pernica JM, Harman S, Kam AJ, Carciumaru R, Vanniyasingam T, Crawford T, et al. Short-course antimicrobial therapy for pediatric community-acquired pneumonia: the SAFER randomized clinical trial. JAMA Pediatr 2021;175:475-82.



52. Shim JY. Current perspectives on atypical pneumonia in children. Clin Exp Pediatr 2020;63:469-76.




53. Lee H, Yun KW, Lee HJ, Choi EH. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev Anti Infect Ther 2018;16:23-34.


54. Korean Academy of Pediatric Allergy and Respiratory Disease, Korean Society of Pediatric Infectious Diseases. Guidelines for treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Seoul (Korea): Korean Academy of Pediatric Allergy and Respiratory Disease, Korean Society of Pediatric Infectious Diseases, 2019.
55. Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018;66:1492-500.


56. Katzen J, Kohn R, Houk JL, Ison MG. Early oseltamivir after hospital admission is associated with shortened hospitalization: a 5-year analysis of oseltamivir timing and clinical outcomes. Clin Infect Dis 2019;69:52-8.


57. Liu JW, Lin SH, Wang LC, Chiu HY, Lee JA. Comparison of antiviral agents for seasonal influenza outcomes in healthy adults and children: a systematic review and network meta-analysis. JAMA Netw Open 2021;4:e2119151.



58. Choi SH, Choi JH, Yun KW. Therapeutics for the treatment of coronavirus disease 2019 in children and adolescents. Clin Exp Pediatr 2022;65:377-86.




59. Choi JH, Choi SH, Yun KW. Risk factors for severe covid-19 in children: a systematic review and meta-analysis. J Korean Med Sci 2022;37:e35.




60. Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015;385:1511-8.

61. Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677-86.


62. Yang EA, Kang HM, Rhim JW, Kang JH, Lee KY. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med 2019;8:726.



63. Sun LL, Ye C, Zhou YL, Zuo SR, Deng ZZ, Wang CJ. Meta-analysis of the clinical efficacy and safety of high- and low-dose methylprednisolone in the treatment of children with severe Mycoplasma pneumoniae pneumonia. Pediatr Infect Dis J 2020;39:177-83.


64. Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 2005;83:353-9.


65. World Health Organization. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. 2nd ed. Geneva (Switzerland): World Health Organization, 2013.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


