Article Contents
| Clin Exp Pediatr > Volume 67(2); 2024 |
|
Abstract
Atopic dermatitis (AD) is a lifelong disease that markedly impairs quality of life. AD is considered a starting point of the ŌĆ£atopic march,ŌĆØ which begins at a young age and may progress to systemic allergic diseases. Moreover, it is strongly associated with comorbid allergic and in’¼éammatory diseases including arthritis and in’¼éammatory bowel disease. Understanding the pathogenesis of AD is essential for the development of targeted therapies. Epidermal barrier dysfunction, immune deviation toward a T helper 2 proin’¼éammatory profile, and microbiome dysbiosis play important roles via complex interactions. The systemic involvement of type 2 in’¼éammation, wheather acute or chronic, and whether extrinsic or intrinsic, is evident in any type of AD. Studies on AD endotypes with unique biological mechanisms have been conducted according to clinical phenotypes, such as race or age, but the endotype for each phenotype, or endophenotype, has not yet been clearly identified. Therefore, AD is still being treated according to severity rather than endotype. Infancy-onset and severe AD are known risk factors leading to atopic march. In addition, up to 40% of adult AD are cases of infancy-onset AD that persist into adulthood, and these are often accompanied by other allergic diseases. Therefore, early intervention strategies to identify high-risk infants and young children, repair an impaired skin barrier, and control systemic in’¼éamation may improve long-term outcomes in AD patients. However, to the best of our knowledge, no study has evaluated the effectiveness of early intervention on atopic march using systemic therapy in high-risk infants. This narrative review addresses the latest knowledge of systemic treatment, including Th2 cytokine receptor antagonists and Janus kinase inhibitors, for children with moderate to severe AD that is refractory to topical treatment.
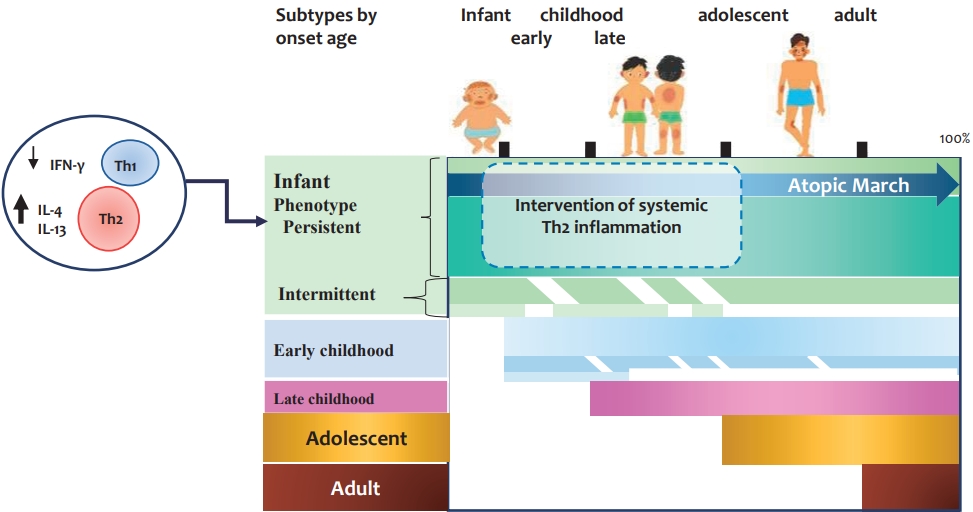
Graphical abstract. Subtypes of adults with atopic dermatitis. Approximately 40%ŌĆō60% of adults with atopic dermatitis develop them in infancy and persist into adulthood. At birth, reduced IFN-╬│- and enhanced IL-4-producing CD4+ cord blood T cells are subsequently associated with infancy-onset atopic dermatitis. Early-onset atopic dermatitis and allergic sensitization at early age increase the risk of the early-onset persistent phenotype. Among infants with early-onset atopic dermatitis, some develop systemic allergic disease such as food allergy, allergic rhinitis, and asthma. The figure is based on the findings described by references 8ŌĆō13, 15, and 16. IL, interleukin; IFN, interferon; Th1, T helper 1; Th2, T helper 2.
Atopic dermatitis (AD) was once considered an early-onset pediatric disease that usually resolves around the age of 2ŌĆō3 years [1]. Although approximately 40%ŌĆō60% of infancy-onset AD cases achieve remission by 6ŌĆō7 years of age, recent studies reported that AD is a lifelong disease with recurrent exacerbations [2-4]. AD is frequently accompanied by systemic allergic diseases, other in’¼éammatory diseases, and/or psychosocial disorders such as depression, anxiety, sleep disorders, and attention deficit hyperactivity disorder [5,6]. Therefore, the burden of AD is quite high. Given the fact that 85% of all AD cases begin within the age of 5 years, this period may be critical not only for AD development but also for disease modification.
Recent studies have provided new insights into the complex pathophysiology and phenotypes of AD. Moreover, based on an extended understanding of its pathogenesis, new agents are being tested in patients with moderate to severe AD. This review describes the latest knowledge about pediatric AD, the pathogenesis and phenotypes of it, and the currently available systemic therapeutics for children with moderate to severe AD that is refractory to topical treatment.
ŌĆ£Atopic marchŌĆØ is the progression of allergic diseases from AD to other immunoglobulin E (IgE)-mediated diseases such as food allergy (FA), allergic rhinitis, and asthma. Over the past 20 years, the term atopic march has been widely used to describe changes in the temporal prevalence of allergic diseases reported in epidemiological studies ranging from AD and FA in infancy to allergic rhinitis and asthma in childhood [7]. These results led to the hypothesis that AD is the first manifestation of an atopic phenotype starting in infancy and early childhood [8]. There is epidemiological and experimental evidence supporting AD as the initiation of allergic diseases [8,9]. Several prospective birth cohorts have shown an association between early-onset AD and the development of asthma and allergic rhinitis at school age [8-10]. The risks of respiratory allergic diseases are greater in children with the early-onset persistent AD phenotype [11]. AD children with specific IgE antibodies (extrinsic AD) by 2ŌĆō4 years of age are at higher risk of the progression of atopic march to allergic rhinitis and asthma than those without (intrinsic AD) [12]. A Canadian birth cohort study reported that a significantly increased risk of FA, asthma and allergic rhinitis was observed in 1-year-old children with AD and allergic sensitization versus those with neither condition [13]. A defective skin barrier, an AD hallmark, has been suggested as a mechanism of atopic march [8,9].
A recent systematic review and meta-analysis of 7 birth cohort studies evaluated AD prevalence across 3 to 6 time points among patients aged 3 months to 26 years and found no significant difference in AD prevalence before versus after childhood [14]. The presence of AD symptoms varied among individuals. Multiple studies found that individuals with early-onset AD were more likely to have symptoms at older ages [14]. The reason for the similar estimated prevalence across ages can be explained by the combination of 3 categories: active disease in both childhood and early adulthood, intermittent disease clearance periods, and later-onset disease.
Two population-based birth cohort studies reported that only a small proportion (~7%) of children with AD experience the complete manifestations of atopic march [15]. However, other studies reported that individuals with early-onset AD are more likely to be symptomatic until later in life, with approximately 17%ŌĆō31% of patients who developed AD by 2 years of age had AD at all time points up to 18 years of age [15,16]. Approximately 40% of adults with AD have infancy-onset disease, 30% have chronic symptoms into adulthood (early-onset persistent phenotype), and 10% have intermittent symptoms (early-onset intermittent phenotype) [16]. Therefore, it is important to identify high-risk infants with AD that will persist into childhood and adolescence. In addition, early intervention is required to modify atopic march (see Graphical abstract). However, to date, there have been no intervention studies aimed at modifying the atopic march in infants with moderate to severe AD.
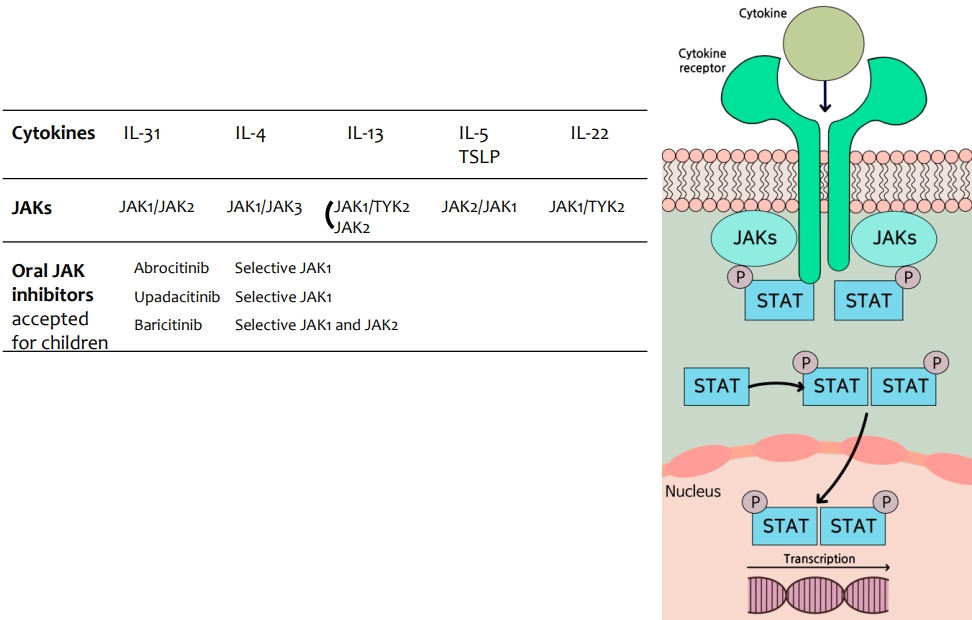
The pathophysiology of AD is complex and multifactorial, caused by interactions between various factors, including epidermal barrier dysfunction, immune dysregulation, microbiome dysbiosis, and pruritus, with strong genetic susceptibility (Fig. 1) [17]. A considerable body of evidence suggests that both epidermal barrier dysfunction and immune deviation to T helper 2 (Th2) in’¼éammation play key roles in AD.
Two hypotheses have been proposed: inside to outside and outside to inside. The first hypothesis is that abnormalities in the innate immune system cause skin in’¼éammation, leading to barrier impairment upon antigen or irritant stimulation. Various mutations and polymorphisms of in’¼éammatory genes have been associated with AD, such as interleukin (IL)-4 receptor ╬▒ and the cluster of differentiation (CD)-14 genes, the serine protease inhibitor Kazal type 5, regulated on activation, normal T cell expressed and secreted (RANTES), IL-4, and IL-13 [18]. Th2 lymphocyte-dominant immune dysregulation produces IL-4 and IL-13, which inhibit filaggrin (FLG) expression.
The outside to inside hypothesis suggests that an impaired skin barrier is the first step in AD pathogenesis and causes immune dysregulation. FLG is an important structural protein in the stratum corneum (SC) that ensures proper epidermal differentiation and skin barrier function [19]. FLG breakdown products produced in the cornified layer contribute to skin moisture retension, pH regulation, barrier permeability regulation, and microbial protection [19,20]. The FLG loss-of-function mutation and its effects on epidermal integrity provide strong evidence supporting outside to inside hypothesis. While there is ongoing debate regarding the sequence of events, it is evident that both epidermal barrier dysfunction and immune dysregulation significantly contribute to the pathogenesis of AD, as they intricately interact with each other.
Recently, it has been suggested that barrier-initiated AD pathogenesis may induce immune dysregulation, further compromising permeability barrier function and forming a potential vicious outside-inside-outside circle in AD.
Although AD is well known to be characterized by a strong Th2 immune response, it is recently recognized as a more heterogeneous disease with additional involvement of the Th22, Th17/IL-23, and Th1 cytokine pathways depending on disease subtype [21].
The epidermal barrier consists of SC and tight junctions, and SC are composed of corneocytes and the extracellular matrix, called the brick and mortar structure. Intact skin is an important physical and immunological barrier to allergens, microbes, and chemicals. Skin barrier impairment, caused by inherited defects or acquired insults, is characterized by downregulated epidermal barrier structural proteins (including FLG, keratins, loricrin, involucrin, and cell adhesion molecules), decreased intercellular lipids and enzymes, decreased antimicrobial peptides (AMPs), increased skin pH, and reduced skin microbiome diversity with a greater abundance of Staphylococcus aureus [21]. Most patients with AD have reduced epidermal terminal differentiation and SC ceramide levels, either primarily or secondarily by immunemediated mechanisms. A disrupted epithelium exposed to stimuli such as proteolytic allergens, bacteria, parasites, and chemicals promotes antigen penetration and triggers a variety of proteinase-activated receptors and pattern recognition receptors on barrier epithelial cells, inducing the release of epithelial-derived cytokines (alarmins) such as thymic stromal lymphopoietin (TSLP), IL-33, and IL-25. In the epithelial regulation of allergic-type 2 responses, 3 epithelial-derived cytokines are critical mediators of type 2 in’¼éammation through the activation of dendritic cells (DCs) and type 2 innate lymphoid cells (ILC2s) [19-23]. DCs at barrier surfaces present processed allergens to naive T cells in the draining lymph nodes through major histocompatibility complex class II molecules. In the presence of IL-4, naive T cells differentiate into Th2 cells, the major cell type that skews the immune reaction to allergens by producing the cytokines IL-4, IL-5, IL-9, IL-13, and IL-31. Activated Th2 cells and ILC2s release IL-4 and IL-13, which promote IgE class switching [19-23]. In addition, IL-4 and IL-13 stimulate keratinocytes to produce TSLP. TSLP overexpression has been identified in the keratinocytes in both acute and chronic lesions of AD [24]. TSLP activates OX40 ligand-expressing dermal DCs to induce differentiation of naive T cells to in’¼éammatory Th2 cells. The epidermal production of TSLP is correlated with clinically observed lesions and AD severity and persistence [24].
Cytokines and chemokines, such as IL-4, IL-5, IL-13, eotaxins, CC chemokine ligand (CCL)17, CCL18, and CCL22, are produced by Th2 cells and DCs and stimulate the infiltration of DCs, mast cells, and eosinophils into the skin. ILC2s are potent sources of IL-5 and IL-13.
IL-22, an ╬▒-helical cytokine belonging to the IL-20 subfamily that is strongly upregulated in AD, is produced by the Th22 cell subset. IL-22 signals via a heterodimer of IL-22 receptor 1 and IL-10 receptor 2, which are expressed on epithelial cells in the skin (keratinocytes), lung, and gut [25]. Increased IL-22 levels act as proin’¼éammatory cytokines, leading to upregulation of AMPs in synergy with IL-17. IL-22 has also been suggested to induce epidermal hyperplasia by promoting keratinocyte proliferation and barrier defects by inhibiting terminal differentiation [26]. IL-22 plays important pathogenic roles in AD initiation and development and is correlated with AD severity.
Th1 and Th17 cells are suggested to play a role, especially in certain subtypes such as intrinsic, pediatric, and Asian phenotypes [27]. However, Th2 and Th22 cells play predominant roles in all AD subtypes [28]. Therefore, dupilumab, which blocks IL-4/IL-13 receptors, is equally effective for extrinsic and intrinsic AD as well as in pediatric and adult AD. Similar or higher Th2 and Th1 activity but much greater Th22 and Th17 immune responses are seen in the lesional skin of patients with intrinsic versus extrinsic AD [29].
Pruritus is the most burdensome symptom in AD, leading to unremitting scratching and further damage to the epithelial barrier, and impairing quality of life of patients and their family. It is primarily a sensory perception of the skin mediated by unmyelinated C-fibers and thinly myelinated A╬┤ fibers originating from cell bodies in the dorsal root ganglion [30]. It has been suggested that endogenous and exogenous pruritogens such as histamine, 5-hydroxytryptamine, proteases, substance P, various cytokines including IL-31 and TSLP, and environmental allergens can signal through specific itch pathways on nerve fiber endings [24,31]. IL-31 is a potent pruritogenic cytokine in AD. Physical damage due to chronic scratching significantly increases cutaneous TSLP levels. TSLP directly causes pruritus as a pruritogen and indirectly by inducing Th2-related cytokines that activate sensory neurons. Moreover, IL-4 enhances neuronal responsiveness to multiple pruritogens. Therefore, pruritogens, including TSLP and Th2 cytokines, are implicated in AD development and aggravation by inducing itching, scratching, and skin barrier dysfunction [24].
Evidence is growing for an important role of the microbiome in AD pathogenesis: specifically, the abundance of S. aureus and relative reduction of commensal organisms that may play a role in regulating growth of S. aureus [32].
AD is a heterogeneous disease with several phenotypes and endotypes characterized by the activation of diverse cytokine signaling pathways, including Th1, Th2, Th22, and Th17 cells, depending on disease subtype. Phenotypes can be classified according to clinical features, such as age, severity, race, and therapeutic response. An endotype is a subtype of a health condition defined by distinct functional or pathobiological mechanisms such as extrinsic/intrinsic AD based on atopic status. The term endophenotype is used to connect the clinical phenotype and mechanical endotype. Defining a distinct endophenotype is a key to determining personalized therapy. Personalized targeted therapy is possible if there is a unique cytokine signature that characterizes an individualŌĆÖs endotype. Studies on endophenotype based on race/ethnicity and age have been conducted. However, additional studies with a greater number of subjects are required to elucidate the characteristics of these subtypes. Here, we describe the subtypes according to age at onset, atopic status, and disease chronicity, which have shown several distinct characteristics.
The clinical AD phenotypes according to age at onset can be clearly defined. Generally, 4 types are classified: infantile (<2 years), early childhood (2ŌĆō6 years), late childhood (6ŌĆō12 years), and adolescence (12ŌĆō18 years) [33,34].
A European birth cohort study revealed that the prevalence of asthma and FA by 6 years of age strongly increased among children with early phenotypes (aged <2 years), especially those with persistent symptoms [35]. Similarly, a recent Korean study of school-aged children and adolescents with AD found that comorbid FA, allergic rhinitis, and asthma as well as inhalant allergen sensitization were more prevalent in infancy-onset (<2 years of age) than childhood-onset AD (Ōēź2 years of age) [34]. While a significant proportion of patients with the early-onset phenotype can reach complete remission before 2 years of age, another proportion, estimated at up to 40%, continues to suffer from the disease over a long period of time [11], and this category of patients may be at high risk for atopic march [36].
As the immune system changes with age, AD in different age groups may present diverse phenotypes and endotypes. Unique cytokine signatures characterizing individual pediatric endotypes may enable age-specific tailored treatment.
The shape and distribution of lesions in AD vary among age groups: cheeks, scalp, and trunk in infants; extensors of limbs in younger children; ’¼éexural distribution of limbs in older children; and additional lichenified lesions on the forehead and neck in adolescents. These changes may be derived from background endotype skewing over time. Therefore, it is crucial to elucidate them to ensure proper treatment.
In addition, even among children of a similar age, the underlying immunological profiles may differ according to atopic status (intrinsic vs. extrinsic), disease duration (acute vs. chronic), severity (mild vs. moderate to severe), and race. However, unlike in adults, making the distinction between extrinsic and intrinsic AD may not be clear in infants and young children because some intrinsic AD cases evolve to the extrinsic type through sensitization.
Studies with peripheral blood samples suggested that infants present overexpression of regulatory T cells and a greater Th17 lineage capacity than adults [37,38]. At birth, immune responses are Th2 polarized, with low Th1/ interferon (IFN)-╬│ levels in healthy newborns and those with AD. The number of cutaneous lymphocyte antigen (CLA)+ Th1 cells was lower in infants and increased with age. Children (<5 years old) with moderate to severe AD showed suppressed and delayed development of skin-homing (CLA+) Th1 cells in the peripheral blood. CLA+ Th1 cell counts in AD infants were lower than those of age-matched controls and older children with AD [39]. However, frequencies of CLA+ Th2 cells were similarly expanded across all age groups of infants, children, adolescents, and adults with AD and significantly higher than in age-matched controls [40]. After infancy, systemic Th2 cells (CLA-Th2 cells) increased in AD patients of all ages, suggesting systemic immune activation with disease chronicity [40]. In addition, IL-22 frequencies also increased from normal levels in infants to significantly higher levels in adolescents and adults versus their respective control subjects. Principal component analysis of the ’¼éow cytometric marker frequencies (percentages) in patients with AD by age showed 3 meaningful age clusters: infants (0ŌĆō5 years), children and adolescents (6ŌĆō17 years), and adults (Ōēź18 years), suggesting unique molecular profiles of AD by age [40].
Epidermal hyperplasia is more common in the lesional skin of children younger than 5 years of age who developed AD within 6 months of age than in adults. In addition, the nonlesional skin of infants and young children shows significant hyperplasia compared to that of adults [41]. Epidermal TSLP expression as early as 2 months of age is associated with AD later in life [42]. Taken together, true AD can be initiated before lesional skin appears in children with early-onset AD.
A study of skin samples taken from moderate to severe AD patients of different ages (0ŌĆō5, 6ŌĆō11, 12ŌĆō17, and Ōēź18 years) found common features of Th2 (Th2-related markers of IL-13, CCL17/thymus and activation-regulated chemokine, CCL18/pulmonary and activation-regulated chemokine, and IL-4R) and Th22 skewing (Th22-related markers of IL22 and S100As) [43]. The differences in expression levels of cytokines between age groups of AD were as follows: infants showed the greatest Th17-related cytokines, whereas long-standing adults displayed Th1 skewing cytokines, including IFN-╬│ and C-X-C motif chemokine ligand (CXCL)9/CXCL10/ CXCL11, suggesting disease chronicity. The expression level of Th17-related genes was inversely related to the developmental age of children aged 0ŌĆō11 years with or without AD, was generally higher in the skin of AD patients versus healthy controls, and presented 2 peaks, with the highest expression in infants followed by adults [43]. Although the role of Th17 in AD has not yet been clearly elucidated, IL-17 is less important in AD than in psoriasis. Table 1 shows the characteristics of subtypes by age at onset.
AD has long been subdivided into extrinsic/atopic and intrinsic/nonatopic subtypes. The extrinsic phenotype (60%ŌĆō80% of cases) is characterized by high serum total and specific IgE levels, eosinophilia, personal and familial atopic backgrounds, and a higher FLG mutation rate. In contrast, patients with intrinsic AD (20%ŌĆō40%) have normal IgE levels, no other atopic background, a female predominance, a delayed disease onset, and preserved barrier function [44,45]. However, even in the same extrinsic subtype, there may be differences in the sensitized allergens as well as stage and site of AD lesion according to age. Sensitization to food allergens is common in infants and young children, whereas sensitization to inhaled allergens is more frequent in older children.
Most studies in infants and young children have attempted to characterize disease phenotypes using peripheral blood analysis. The eosinophil count, eosinophil cationic protein level, and IL-5 detection rate were higher in infants with extrinsic versus intrinsic AD [46].
Increased Th1 signals (IFN-╬│, CXCL9, CXCL10, and MX-1) and more pronounced Th17/Th22 activation (IL-17A, CCL20, Elafin, and IL-22), which are linked to psoriasis, are noted in intrinsic AD. Levels of antimicrobial activity (S100A9 and S100A12), which are coregulated by IL-17/IL-22, are higher in intrinsic versus extrinsic lesions [47].
In the skin, Th1/IFN-╬│-related gene expression and levels of the Th17 chemokine CCL20 are correlated with disease severity in intrinsic AD. On the other hand, Th2 markers were positively correlated with disease severity but negatively correlated with the barrier products of loricrin, periplakin, and FLG in extrinsic AD [46]. Intrinsic AD has in’¼éammatory (IL-22, IL-36╬▒/╬│, IL-36RN, and CCL22) and lipid metabolism pathways that overlap with psoriasis, supporting Th17/IL-23 and IL-22 as common profiles of both conditions [47].
Although each type has characteristic cytokine profiles, they share a similar clinical presentation in the lesional skin and a similar increase in Th2 markers; increased infiltration of T cells and DCs in the lesional and nonlesional skin of both AD (more cellular infiltrates of T cells, myeloid DCs, and Langerhans cells in intrinsic AD) and epidermal hyperplasia in the lesional skin versus nonlesional skin [47]. Table 2 summarizes the characteristics of subtypes by atopic status.
Skin lesions vary widely, but can be classified as acute or chronic. Acute lesions begin with erythematous papules and serous exudates with intense itching and include secondary lesions, such as excoriations and crusted erosions due to scratching. When acute lesions persist, subacute lesions such as erythematous scaly papules and plaques appear. Progressive itching and rashes result in chronic lichenified lesions characterized by prominent skin marks with hyper- or hypopigmentation.
AD usually presents as multiple lesions of different stages at multiple sites. It is common to find overlapping acute and chronic lesions in the same patient. Acute lesions begin with a marked increase in AMP and a lesser increase in IL-17 levels as well as the upregulation of Th2 and Th22 cytokines. Intensification of the Th2 and Th22 cytokine axes with disease chronicity has been demonstrated along with significant increases in Th1 [48,49]. Taken together, acute in’¼éammation in AD is driven by type 2 cytokines, while enhanced Th2 and Th22 as well as additional Th1 responses are involved in the chronic stage; changes from acute to chronic AD are quantitative rather than qualitative in terms of Th2, Th22, Th1, and Th17 responses, and additional features develop only in chronic in’¼éammation (Table 3) [48,49].
Most patients with mild to moderate AD respond to standard topical anti-in’¼éammatory therapies with optimized skincare. Nevertheless, it is often inadequately controlled by the avoidance of irritants or triggers (food and environment), application of emollients, and intensive topical treatments. The importance of treating children with AD comes from the fact that up to 80% of cases begin in infancy or early childhood and AD is an early presentation of the allergic march. Therefore, it is ideal to shift the treatment goal from symptom resolution to modulation of the immune response behind it. Therefore, early systemic treatment requires in young children with immune dysregulation. However, treatment options for this age group are limited due to the lack of the clinical trial data on the effectiveness and long-term safety of new agents. In addition, no clinical studies have determined whether early systemic treatment of immune dysregulation can modify the disease course in infants and young children. Here, we present a descriptive review of currently accepted new systemic therapies, Th2 cytokine receptor antagonists and Janus kinase inhibitors (JAKi), for children with moderate to severe AD. The results of new systemic agents other than those approved in pediatric AD are not discussed here.
IL-4, IL-13, IL-5, and their respective receptors have been targets of drug development strategies to modulate the Th2 response, a core pathway in AD [50]. IL-4 and IL-13 receptors are expressed in neurons and believed to play additional roles in the itch-scratch mechanism [51].
Dupilumab is a human monoclonal antibody that inhibits downstream signaling of IL-4 and IL-13 by binding to IL4R╬▒ [50]. IL-4 and IL-13 share a heterodimeric receptor composed of IL-4R╬▒ and IL-13R╬▒1, known as the type 2 receptor of IL-4 [52]. It is approved (2022, U.S. Food and Drug Administration; 2022, Korean Ministry of Food and Drug Safety) for children aged 6 months and older with moderate to severe AD. Phase 3 studies evaluated the efficacy and safety of dupilumab for the treatment of moderate to severe AD in children and adolescents aged 6 months to 17 years and reported improvement in AD signs and symptoms, including itching, sleep loss, and quality of life (Table 4, Figs. 2 and 3) [53-55]. Adolescents were administered dupilumab (200/300 mg every 2 weeks or 300 mg every 4 weeks) for 16 weeks. The proportion of patients with 75% improvement in the eczema area and severity index score (EASI 75) from baseline was 41.5%, 38.1%, and 8.2% on 2-week and 4-week dupilumab and placebo regimens, respectively (Table 4, Fig. 2) [53]. In a phase 2a open-label sequential cohort study with a phase 3 open-label extension, adolescents with moderate to severe AD were administered dupilumab 2 mg/kg or 4 mg/kg on a weekly basis. The percent change in EASI from baseline to week 52 for the 2 mg/kg and 4 mg/kg regimens was -85% and -84%, respectively [56]. Almost all children reported at least one mild to moderate treatment-emergent adverse event (TEAE) with a dose-related trend; none led to interruption of treatment.
In children aged 6ŌĆō11 years, the administration of dupilumab with a weight-based regimen of 100/200 mg every 2 weeks (q2 wk) or 300 mg every 4 weeks (q4 wk) for 16 weeks combined with a medium-potency topical corticosteroid (TCS) improved AD; the proportions of patients who achieved an EASI 75 in q2w and q4w dupilumab regimens and placebo were 58.3%, 50.8%, and 12.3%, respectively (Table 4, Fig. 2) [54].
Similar results were reported for younger age groups (6 months to <6 years) with moderate to severe AD. They were given 200/300 mg of dupilumab every 4 weeks for 16 weeks combined with TCS; more patients treated with dupilumab than placebo achieved an EASI 75 (53% vs. 11%) and InvestigatorŌĆÖs Global Assessment (IGA) score 0/1 (clear/ almost clear, 28% vs. 4%) (Table 4, Fig. 2) [55]. Itching, one of bothersome symptom, was also remarkably improved in the dupilumab group; the percentage change from baseline in worst itch Numerical Rating Scale score was -49.4% and -2.2% in the dupilumab and placebo groups, respectively. The incidence of conjunctivitis was higher in the dupilumab versus placebo group (5% vs. 0%). However, skin infections, excluding herpes virus infections, were less frequent in the dupilumab versus control group [55].
An ongoing open-label extended phase 3 study (52 weeks) is evaluating the efficacy and long-term safety of dupilumab in patients with moderate to severe AD aged Ōēź6 months to <18 years participating in 3 parent studies [53,56-58]. Results of dupilumab in adolescents (Ōēź12 to <18 years) among enrolled subjects were recently reported [58]. Patients enrolled in the 3 parent studies and newly enrolled patients received 300 mg of dupilumab every 4 weeks regardless of body weight during the extended study period under the new protocol. At week 52, 42.7% of patients achieved an IGA score of 0/1, while 81% achieved an EASI 75 [58].
Adverse reactions of special interest were mild to moderate and resolved with continued treatment. Clinical trials have reported an increased incidence of conjunctivitis with dupilumab versus placebo. Interestingly, it occurs more frequently in patients with AD versus other diseases such as asthma, chronic rhinosinusitis with nasal polyps, or eosinophilic esophagitis [53,59]. Dupilumab-associated conjunctivitis is less common in children versus adults [60]. However, the exact pathophysiology of conjunctivitis remains unclear.
In summary, dupilumab has a favorable safety profile, even in 6-month-old children and can be administered as a long term therapy in pediatric AD.
Selective IL-13 antagonists such as lebrikizumab and tralokinumab can manage AD and improve patientsŌĆÖ quality of life [61]. The 2 agents differ in their binding epitopes and ability to block one or both IL-13 receptors; lebrikizumab does not block 13╬▒2 receptor chains, whereas tralokinumab blocks binding of IL-13 to the IL-13R╬▒1 and IL-13╬▒2 receptor chains, the decoy receptor, which may be involved in endogenous IL-13 regulation [61].
Tralokinumab is a fully humanized antibody targeting IL-13 that blocks its binding to both IL-13R╬▒1 and IL-13╬▒2 receptor chains [62,63]. It has been approved for the treatment of moderate to severe AD in adults after being studied for up to 52 weeks in phase 3 studies [64,65]. Significant improvements in AD assessment scores, pruritus, sleep interference, and quality of life were noted and maintained over time without the requirement for TCS [64,65]. The results of a phase 3 trial for tralokinumab monotherapy in adolescents (aged 12ŌĆō17 years) were released [66]. Tralokinumab (150 or 300 mg) was administered every other week; EASI 75 was above 25% by the end of week 16 and reached 44%ŌĆō64% by the end of maintenance treatment at week 52 with a favorable safety profile. The proportion of patients who achieved an IGA score of 0/1 in the 150 mg and 300 mg tralokinumab and placebo groups was 21.4%, 17.5%, and 4.3%, respectively. The proportion of patients who achieved an EASI 75 in the 150 mg and 300 mg tralokinumab and placebo groups was 28.6%, 27.8%, and 6.4%, respectively (Table 4, Fig. 3). Upper respiratory tract infection was the most common TEAE during the maintenance and safety follow-up period (Table 4) [66].
Lebrikizumab is a fully humanized anti-IL-13 antibody that specifically binds to soluble IL-13 and does not block cytokine binding to the receptor but impairs the heterodimerization of IL-4R╬▒ and IL-13R╬▒1, thereby inhibiting signal transduction [62,67]. Multicenter double-blind placebo-controlled phase 3 randomized clinical trials evaluated the efficacy of lebrikizumab as monotherapy (ADvocate 1 and 2) and a combination therapy with TCS (Adhere) in the treatment of adolescents (aged Ōēź12 to <18 years; weight, Ōēź40 kg) and adults with moderate to severe AD [67,68]. At week 16, EASI 75 in the treatment group vs. placebo was 58.8% vs. 16.2%, 52.1% vs. 18.1% and 69.5% vs. 42.2% in ADvocate1, ADvocate2 and Adhere trials, respectively [67,68]. The TEAEs were mild or moderate in severity and did not lead to discontinuation of the study. The most frequently reposted TEAEs were conjunctivitis (7.4%, 7.5%, 4.9%) and headache (3.2%, 5%, 4.8%) herpes infection (3.2%, 2.8%, 3.4%) in ADvocate1, ADvocate2 and Adhere trials, respectively (Table 4, Fig. 3) [67,68]. A 52-week open label study evaluated the efficacy and safety of lebrikizumab exclusively in adolescent patients of ADvocate1, ADvocate2 and Adhere trials [69]. Serious events leading to treatment discontinuation were infrequent, and 81.9% achieved an EASI 75 by Week 52 [69].
In adults, the improvement in EASI scores after adjusting for placebo was comparable between dupilumab and lebrikizumab (32%ŌĆō36% and 37%, respectively) and slightly lower in tralokinumab (12%ŌĆō22%), which may be attributed to differences in the study designs, but because tralokinumab also blocks receptors involved in the endogenous regulation of IL-13 [61,70].
Nemolizumab is a humanized monoclonal antibody against receptor A of IL-31, a prominent pruritogenic cytokine produced by infiltrating Th2 cells in AD, which correlates with disease severity and has been found to be excessively expressed in skin lesions in AD [31,71]. Therefore, IL-31 and its receptor are the focus of strategies to better control the itch-scratch cycle [72,73]. It has been approved for moderate to severe AD over the age of 13 years in adolescents and adults in Japan. In a double-blind, phase 3 trial, 60 mg nemolizumab was subcutaneously administered every 4 weeks with concomitant topical agents to subjects aged 13 years or older with moderate to severe pruritus unresponsive to topical agents, and adolescents aged 13ŌĆō17 years accounted for approximately 5 percent of subjects [74]. After week 16, the mean percent change in the EASI score was -45.9% with nemolizumab and -33.2% with placebo (Table 4, Fig. 3). Adverse events were generally mild to moderate; however, 1 in the nemolizumab groups discontinued treatment due to AD exacerbation [74]. Although some patients reported exacerbation of AD as an adverse event, these patients also experienced less itching as measured by visual analog scale score [74].
In an open-label phase 2 study of patients aged 12ŌĆō17 years with moderate to severe AD, nemolizumab was administered subcutaneously as a 60-mg loading dose, followed by 30 mg every 4 weeks until 12 weeks and followed up for 8 more weeks [75]. AD-related proin’¼éammatory biomarkers changed more significantly in EASI responders than in EASI nonresponders [73].
As nemolizumab appears to be a promising agent, large-scale studies are required to evaluate its long-term efficacy and safety. A phase 2 study is ongoing to evaluate the pharmacokinetics, safety, and efficacy of nemolizumab for moderate to severe AD in children aged 2ŌĆō11 years (NCT04921345).
Janus kinase (JAK)s are a group of molecules comprising JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). Binding of different cytokines to their specific receptor subunits on different cell populations leads to the activation of a specific JAK-signal transducers and activators of transcription (STATs) pathway, and the different isoforms of JAKs are coupled to specific receptor/cytokine pairs. When a cytokine binds to its intracellular domains of type I or type II cytokine receptors, a conformational change is induced, which activates JAK-tyrosine kinases, resulting in phosphorylation of tyrosine residues in the receptor's intracellular domain [40,76]. The phosphorylation of receptor subunits allows for the recruitment of signal molecules, including latent cytoplasmic transcription factors such as STATs, phosphorylated STATs are activated, dimerized, and translocated to the nucleus to regulate target gene expression (Fig. 4). In general, all type I and II receptors rely on JAK1/JAK2 for signal transduction. Depending on the particular receptor, one or more members of the JAK family work together to mediate signal transduction. Therefore, each JAK is often involved in the downstream signaling of multiple cytokine receptors in association with other JAK family members. TYK2 can partner with both JAK1 and JAK2, whereas is a much less widely expressed JAK protein and restricted to receptors containing the common ╬│ chain-containing receptors (Fig. 4).
JAK inhibitors (JAKi) are small molecules that can be administered orally or topically and are recently introduced to treat AD. Currently, more than 90 JAKi are patented, many of which are in clinical development for various indications, such as in’¼éammatory bowel diseases and rheumatoid arthritis [43,76]. As the binding of Th2 and Th22 cytokines to their receptors involves downstream JAK-STAT signaling, JAKi are emerging as attractive compounds for AD treatment.
JAKi approved or under clinical development for AD can be classified into 3 main categories: nonselective (delgocitinib, cerdulatinib, jaktinib, CEE321), dual (baricitinib, ruxolitinib, brepocitinib, ATI-1777), and selective (upadacitinib, abrocitinib, SHR0302) [77].
JAK inhibition exerts a broad immunopharmacological effect by blocking the signal transduction pathways of multiple cytokines. JAKi blocks numerous cytokines that are involved in many aspects of host defense, hematopoiesis, metabolism, cell growth, and cell differentiation; therefore, they can have multiple systemic effects [78]. Serious adverse effects include infections, anemia, pulmonary embolism, malignancy risk, thromboembolic risk, and elevated serum cholesterol [78]. Nonetheless, the hope is a second-generation JAKi with increased selectivity to reduce adverse effects and preserve efficacy. Here, we discuss only orally available JAKi that have been studied in pediatric patients (Table 4, Fig. 3).
Baricitinib is a selective JAK1/JAK2 inhibitor that has been approved for the treatment of patients with moderate to severe AD aged 12 years and older. In 2 independent 16-week phase 3 trials, the participants who achieved EASI 75 reached 24.8% with baricitinib monotherapy and 36.6% with baricitinib plus TCS [79]. The most common adverse events were nasopharyngitis and headache [79]. No cardiovascular disease, venous thromboembolism, or serious hematological changes were detected during the 16-week treatment period. Unlike selective JAK1 inhibitors, no increase in acne incidence was noted [79]. In a pooled safety analysis of cumulative data from 8 adult studies (n= 2,531), simple viral infection and headache were the most frequently reported TEAE, with 2 major cardiovascular events, 2 venous thrombosis events, and 1 death [80]. One death reported after taking baricitinib for more than 12 months was due to gastrointestinal bleeding. The patient was randomized to 1 mg in the first study and then to 4 mg, which was reduced to a renally adjusted dose of 2 mg because of reduced glomerular filtration rate in the long-term extension study. With the satisfactory effect of improvement and onset of action as well as an acceptable safety profile, baricitinib has been studied in children aged 2ŌĆō17 years with an inadequate response to topical treatment (Table 4) [81]. The baricitinib 4-mg equivalent for 16 weeks achieved a significant improvement in EASI and itching versus placebo.
As hematopoietic signaling of receptors depends crucially on JAK2 homodimers, JAK1 selective inhibitors are suggested as a safer option to avoid major JAKi adverse effects. Upadacitinib and abrocitinib are second-generation JAK1 inhibitors that have been studied in children. Upadacitinib is a selective JAK1 inhibitor approved for the treatment of moderate to severe AD in adults and children aged 12 years. Three phase 3 trials (Measure Up 1, Measure Up 2, and AD Up) evaluated the efficacy of upadacitinib for 16 weeks (15 or 30 mg, once daily) in the treatment of patients aged 12 years or older with moderate to severe AD [82,83]. Measure Up 1 and 2 evaluated upadacitinib as monotherapy, while AD Up examined it with TCS for all participants. The percentage of participants who achieved EASI 75 in Measure Up 1, Measure Up 2, and AD Up was satisfactory with the 15 mg dose (69.6%, 60.1%, and 65 %, respectively), and 30 mg dose (79.7%, 72.9%, and 77%, respectively); both regimens showed statistically significant improvements by week 2. Adverse reactions were mild to moderate in severity and included acne, upper respiratory tract infection, headache, oral herpes, and asymptomatic elevation of plasma creatine phosphokinase. Acne was the most common side effect in all 3 studies. Although acne was not serious and did not lead to treatment discontinuation, it was higher than that observed in previous studies of rheumatoid arthritis, which may be due to the relatively younger age of patients with AD (Table 4) [84,85]. These results demonstrate the potential of upadacitinib as a monotherapy to reduce the burden of TCS use [82,83]. In addition, the incidence of oral herpes infection was lower in upadacitinib monotherapy (3%) than in combination therapy with upadacitinib and TCS [82,83].
Upadacitinib efficacy at week 16 was maintained through the 52-week follow-up studies [86,87]. EASI 75 was achieved in 82.0%, 79.1% and 50.8% of patients maintained on the 15 mg dose and in 84.9%, 84.3% and 69.0% of patients maintained on the 30 mg dose in the Measure Up 1, Measure Up 2 and AD UP studies, respectively. Both doses of upadacitinib were well tolerated, with no new critical safety issues, and a very low rate of treatment discontinuation due to adverse events [86,87]. An open-label multiple dose study in the younger age group (2 to <12 years of age) is currently in progress (NCT 03646604).
Abrocitinib is a JAK1 selective inhibitor that has been approved for the treatment of moderate to severe AD in adolescents and adults. Three phase 3 trials evaluated the efficacy and safety of once-daily 100 mg or 200 mg of abrocitinib for 12 weeks; JADE Mono 1 and 2 studied abrocitinib monotherapy in adolescents and adults (adolescents were 22% and 10% of the study subjects, respectively), whereas JADE TEEN examined abrocitinib plus TCS in adolescents (Table 4, Fig. 3) [88-90].
With the 100-mg dose, the percentage of participants achieved EASI 75 in JADE Mono 1 and 2 as well as JADE TEEN was 40%, 44.5%, and 68.5%, respectively and with 200 mg dose, it was 63%, 61%, and 72%, respectively. Patient-reported signs and symptoms, including sleep loss and quality of life, were substantially improved with abrocitinib monotherapy or combination therapy compared to placebo in adolescents enrolled in JADE TEEN as well as JADE Mono 1 and 2 [90]. Commonly reported adverse events were nausea, vomiting, abdominal pain, headache, increased blood creatine phosphokinase, and acne [88-90]. Thrombocytopenia was noticed in 3 studies and resolved with continued treatment, except for 1 patient who had to withhold treatment for 8 days [88-91]. Regarding the serious side effects of JAKi, no thromboembolism or major cardiovascular events were reported. In upadacitinib studies, the elevation of liver enzymes in the Measure Up 1 and 2 groups and placebo group was 1.7% and 1.1%, respectively, which did not lead to discontinuation of treatment. An integrated safety analysis of a phase 2b study, 4 phase 3 studies and 1 long-term extension study was conducted to evaluate the long-term safety of abrocitinib in adolescents, who represented only 12.7% of all patients in the abrocitinib group [92]. Four events of herpes zoster (0.2%; all in the abrocitinib 200 mg group) resulted in permanent discontinuation of study treatment; abrocitinib 200 mg, age Ōēź65 years, and severe disease at baseline were associated with higher risk of herpes zoster [92]. Incidence rates presented as numbers of patients with events per 100 patient-years (PYs) were 2.33/100 PY and 2.65/100 PY for serious infection, 4.34/100 PY and 2.04/100 PY for herpes zoster, and 11.83/100 PY and 8.73/100 PY for herpes simplex in the 200-mg and 100-mg groups, respectively. Five venous thromboembolism events occurred (IR 0.30/100 PY) in the 200-mg group [92].
Comparative studies were performed between selective JAKi and dupilumab in adults [93,94]. Upadacitinib 30 mg and abrocitinib 200 mg were superior to dupilumab 300 mg in terms of onset of itch improvement and EASI 75 at 16 weeks. The adverse effects of the 3 drugs were consistent with those reported in previous studies and were mild to moderate in severity. The risk of adverse events was numerically higher in the upadacitinib 30 mg and abrocitinib 200 mg groups than in the dupilumab 300 mg group; however, serious adverse events during treatment were similar across study groups [93,94]. Each drug has its own characteristics including pharmacokinetics. Therefore, this finding needs to be carefully interpreted. JAKi are administered orally every day, and their efficacy is maintained constantly, whereas dupilumab is injected subcutaneously at 4-week intervals; therefore, the concentration before administration is relatively low, and the main outcomes were measured at 4-week intervals, except for weeks 1 and 2.
AD is a heterogeneous systemic in’¼éammatory skin disease associated with immune dysregulation, epithelial barrier dysfunction, and pruritus. Approximately 60%ŌĆō80% of adults with AD develop the disease during the first 2 years, and 85% develop the disease before 5 years of age, although the rates vary between studies. Up to 40% of patients with infancy-onset AD suffer from the disease into adulthood, and some progress to the atopic march. In addition to concerns about AD chronicity, the systemic Th2 in’¼éammatory response in moderate to severe AD, even at a young age, indicates the need for early appropriate systemic treatment. However, for this very important period in young children, we have limited options for disease intervention. Fortunately, dupilumab, an IL-4 and IL-13 antagonist, has recently been approved for use in children aged 6 months and older. Currently, it is time to focus on whether early treatment for high-risk infants and young children can modify the disease course. For this purpose, background immunologic profiles and clinical features including skin characteristics in young children with AD should be further elucidated.
Fig.┬Ā1.
Pathophysiology of atopic dermatitis. The pathophysiology of atopic dermatitis is complex and multifactorial and caused by the interaction among epidermal barrier dysfunction, immune dysregulation, itching, and microbiome dysbiosis. An impaired epidermal barrier is characterized by downregulated epidermal barrier structural proteins, intercellular lipids and enzymes, decreased AMPs, increased skin pH, and reduced skin microbiome diversity with a greater abundance of Staphylococcus aureus. As a result, antigens can easily penetrate, transepidermal water loss is increased, and epithelial-derived cytokines (alarmins) such as TSLP, IL-33, and IL-25 are released. Epithelial-derived cytokines are critical mediators of type 2 inflammation through activation of DCs and ILC2s. TSLP activates OX40 ligand-expressing dermal DCs to induce naive T cells to differentiate into inflammatory Th2 cells that produce IL-4, -5, -13, and -31. Th2 cytokines including IL-31 and TSLP are potent pruritogens. Th2 and Th22 cells play a major role in AD, and Th1 and Th17 cells have been suggested to play roles as well. AMPs, antimicrobial peptides; Ag, antigen; TEWL, transepidermal water loss; TSLP, thymic stromal lymphopoietin; IL, interleukin; DC, dendritic cells; ILC2, type 2 innate lymphoid cells; Th1, T helper 1; Th2, T helper 2; IgE, immunoglobulin E.
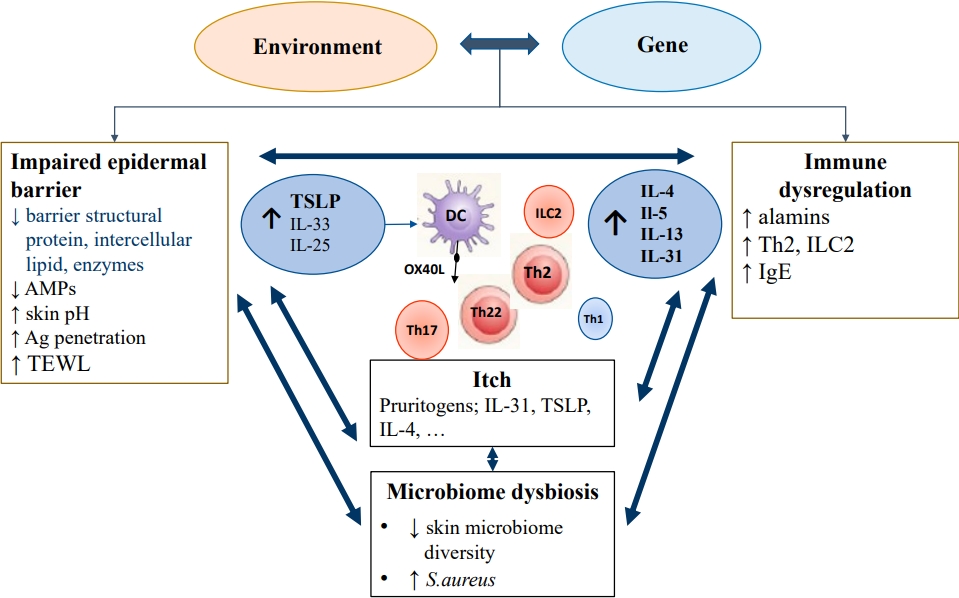
Fig.┬Ā2.
Proportion of moderate to severe atopic dermatitis children aged 6 months to 17 years who achieved an EASI 75 on dupilumab treatment. The figure shows the 3 blinded placebo controlled studies that evaluated the efficacy of dupilumab treatment for 16 weeks in children aged 6 months to 17 years. The label on each bar shows the treatment regimen. q2 wk, every 2 weeks; q4 wk, every 4 weeks; EASI 75, 75% reduction in eczema area and severity index.
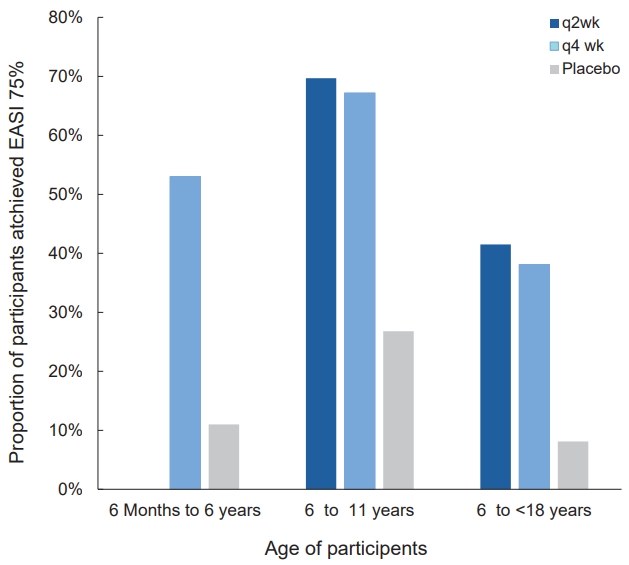
Fig.┬Ā3.
Forest plot of proportion of adolescents with moderate to severe atopic dermatitis who achieved EASI 75 for each treatment. The figure shows the odds ratio and 95% confidence interval (CI) for patients who achieved an EASI 75 in studies evaluating the efficacy of biologics and Janus kinases in adolescents treated for 12ŌĆō16 weeks. *Adult studies included 10%ŌĆō22% of adolescents. q2 wk, every 2 weeks; q4 wk, every 4 weeks; AD, atopic dermatitis; EASI 75, 75% reduction in eczema area and severity index; TCS, topical corticosteroid.
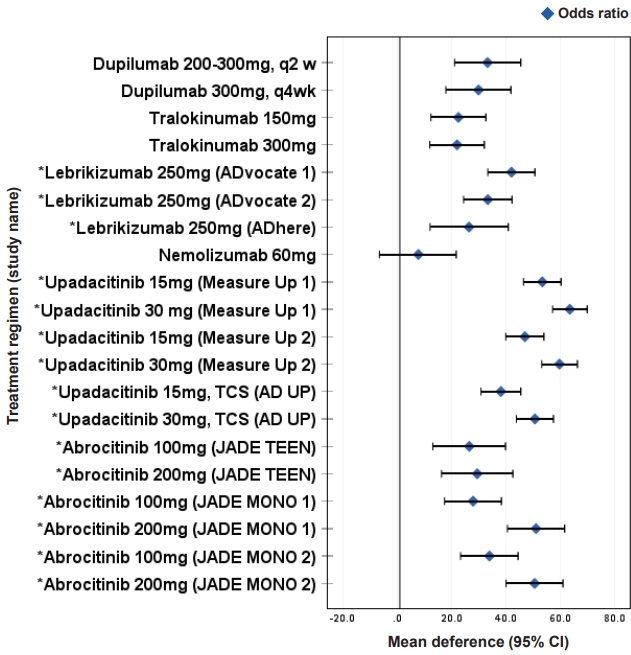
Fig.┬Ā4.
JAK-STAT pathway and JAKs paired with cytokine receptors in atopic dermatitis. When a cytokine binds to the intracellular domains of cytokine receptors, a conformational change is induced and JAK-tyrosine kinases are activated, resulting in the phosphorylation of tyrosine residues in the receptorŌĆÖs intracellular domain. The phosphorylation of receptor subunits allows the recruitment of STATs. Phosphorylated STATs are activated, dimerized, and translocated to the nucleus to regulate the expression of target genes. JAK, Janus kinases; STAT, signal transducers and activators of transcription.
Table┬Ā1.
Phenotypes of atopic dermatitis by age of onset
Table┬Ā2.
Phenotypes of atopic dermatitis by atopic status
Table┬Ā3.
Phenotypes of atopic dermatitis by stage
Table┬Ā4.
Summary of phase 3 studies for the use of systemic biologics and JAKi for AD treatment in pediatrics age
| Medication (mechanism) | Age (yr) (n) | Study design (duration, wk) | Regimen (mg) | Patients achieved EASI 75 (%) | TEAEs higher in treatment groups References | |
|---|---|---|---|---|---|---|
| Dupilumab (IL-4/IL-13 antagonist) | 12ŌĆō<18 (251) | Blinded | q2 wk: | q2 wk: 41.5% | Ōēź5% of patients: conjunctivitis, injection site reaction. | [53] |
| Monotherapy (16) | 200 mg <60 kg | q4 wk: 38.1% | ||||
| 300 mg Ōēź60 kg | Placebo: 8.2% | |||||
| q4 wk: 300 mg | ||||||
| 6ŌĆō11 (367) | Blinded | q2 wk: | q2 wk: 67.2% | Ōēź5% of patients: conjunctivitis, injection site reaction | [54] | |
| Plus TCS (16) | 100 mg <30 kg | q4 wk: 69.7% | ||||
| 200 mg >30 kg | Placebo: 26.8% | |||||
| q4 wk: 300 mg | ||||||
| 6 MonthsŌĆō6 years (162) | Blinded | q4 wk: | Tx: 53% | Conjunctivitis and herpes viral infection | [55] | |
| Plus TCS (16) | 200 mg <15 kg | Placebo: 11% | ||||
| 300 mg Ōēż15 kg | ||||||
| Tralokinumab (IL-13 antagonist) | 12ŌĆō17 (301) | Blinded | q2 wk: | 150 mg: 28.6% | Ōēź 5% of patients : upper respiratory tract infection, injection site reaction | [66] |
| Monotherapy (16) | 150 mg | 300 mg: 27.8% | ||||
| 300 mg | Placebo: 6.4% | |||||
| Placebo | ||||||
| Lebrikizumab (IL-13 antagonist) | 12ŌĆōAdulta) | Blinded | q2wk: 250 mg | ADvocate1 | Ōēź3% of patients: conjunctivitis, headaches, herpes infection | [67] |
| ADvocate1 (424) | Monotherapy (16) | Tx: 58.8% | ||||
| Placebo | Placebo: 16.2% | |||||
| ADvocate2 (427) | ADvocate2 | |||||
| Tx: 52.1% | ||||||
| Placebo: 18.1% | ||||||
| 12ŌĆōAdulta) | Blinded | q2wk: 250 mg | Tx: 69.5% | Ōēź3% of patients: conjunctivitis, headaches, herpes infection | [68] | |
| ADhere (211) | Plus TCS (16) | Placebo: 42.2% | ||||
| Nemolizumab (IL-31 antagonist) | 13ŌĆō18 (143) | Blinded | q4 wk: 60 mg | Tx: 25.9% | Ōēź3% of patients: Injection site reaction, abnormal cytokine, increased blood creatine kinase | [74] |
| Concomitant topical Tx. (16) | Placebo: 18.1% | |||||
| Baricitinib (JAK 1/2 inhibitor) | 12ŌĆō<18 | Blinded | Once daily | 1 mg: 32.2% | Ōēź5% of patients: Abdominal pain, acne, headache | [81] |
| Concomitant topical Tx. (16) | 1 mg | 2 mg: 40.0% | ||||
| 2 mg | 4 mg: 52.5% | |||||
| 4 mg | Placebo: 32.0% | |||||
| Placebo | ||||||
| Upadacitinib (JAK 1 inhibitor) | 12ŌĆō75 | Blinded | Once daily | Measure Up 1 | Ōēź5% of patients: acne, upper respiratory tract infection, headache, increased plasma creatine phosphokinase | [82] |
| Measure Up 1 (847) | Monotherapy (16) | 15 mg | 15 mg: 69.6% | |||
| 30 mg | 30 mg: 79.7% | |||||
| Measure Up 2 (836) | Placebo | Placebo: 16.3% | ||||
| Measure Up 2 | ||||||
| 15 mg: 60.1% | ||||||
| 30 mg: 72.9% | ||||||
| Placebo: 13.3% | ||||||
| 12ŌĆō75 | Blinded | Once daily | 15 mg: 65% | Ōēź5% of patients: acne, oral herpes, increased blood creatine phosphokinase | [83] | |
| AD Up (901) | Plus TCS (16) | 15 mg | 30 mg: 77% | |||
| 30 mg | Placebo: 26% | |||||
| Placebo | ||||||
| Abrocitinib (JAK 1 inhibitor) | 12ŌĆōAdulta) | Blinded | Once daily | 100 mg: 40% | Ōēź 3 % of patients: GIT upset, headache | [88] |
| JADE MONO 1 (387) | Monotherapy (12) | 100 mg | 200 mg: 63% | |||
| 200 mg | Placebo: 12% | |||||
| Placebo | ||||||
| 12ŌĆōAdulta) | Blinded | Once daily | 100 mg: 44.5% | Ōēź3% of patients: GIT upset, acne, headache, increased blood creatine phosphokinase , thrombocytopenia. | [88] | |
| JADE NONO2 (391) | Monotherapy (12) | 100 mg | 200 mg: 61.0% | |||
| 200 mg | Placebo: 10.4% | |||||
| Placebo | ||||||
| 12ŌĆō<18 | Blinded | Once daily | 100 mg: 68.5% | Ōēź3% treatment of patients: GIT upset, acne, increased blood creatine phosphokinase. | [90] | |
| JADE TEEN (285) | Concomitant topical Tx. (12) | 200 mg | 200 mg: 72.0% | |||
| 100 mg | Placebo: 41.5% | |||||
| placebo | ||||||
JAKi, Janus kinase inhibitors; AD, atopic dermatitis; EASI 75; 75% reduction in eczema area and severity index; TEAE, treatment-emergent adverse event; IL, interleukin; q wk, every week; q2 wk, every 2 weeks; q4 wk, every 4 weeks; Tx, treatment; TCS, topical corticosteroid; GIT, gastrointestinal tract.
References
1. Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res 2015;7:101ŌĆō5.




2. Somanunt S, Chinratanapisit S, Pacharn P, Visitsunthorn N, Jirapongsananuruk O. The natural history of atopic dermatitis and its association with Atopic March. Asian Pac J Allergy Immunol 2017;35:137ŌĆō43.


3. Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol 2014;150:593ŌĆō600.



4. Abuabara K, Margolis DJ. Do children really outgrow their eczema, or is there more than one eczema? J Allergy Clin Immunol 2013;132:1139ŌĆō40.



5. Kage P, Simon JC, Treudler R. Atopic dermatitis and psychosocial comorbidities. J Dtsch Dermatol Ges 2020;18:93ŌĆō102.


6. Paller A, Jaworski JC, Simpson EL, Boguniewicz M, Russell JJ, Block JK, et al. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol 2018;19:821ŌĆō38.



8. Tham EH, Leung DY. Mechanisms by which atopic dermatitis predisposes to food allergy and the atopic march. Allergy Asthma Immunol Res 2019;11:4ŌĆō15.




9. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy 2014;69:17ŌĆō27.


10. Saunes M, ├śien T, Dotterud CK, Romundstad PR, Storr├Ė O, Holmen TL, et al. Early eczema and the risk of childhood asthma: a prospective, population-based study. BMC Pediatr 2012;12:168.




11. Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, Dybuncio A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol 2013;110:24ŌĆō8.


12. Novembre E, Cianferoni A, Lombardi E, Bernardini R, Pucci N, Vierucci A. Natural history of "intrinsic" atopic dermatitis. Allergy 2001;56:452ŌĆō3.


13. Tran MM, Lefebvre DL, Dharma C, Dai D, Lou WY, Subbarao P, et al. Predicting the atopic march: results from the Canadian Healthy Infant Longitudinal Development Study. J Allergy Clin Immunol 2018;141:601ŌĆō7.

14. Abuabara K, Yu AM, Okhovat JP, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy 2017;73:696ŌĆō704.




15. Belgrave DC, Granell R, Simpson A, Guiver J, Bishop C, Buchan I, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med 2014;11:e1001748.



16. Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 2013;68:498ŌĆō506.


17. Ratchataswan T, Banzon TM, Thyssen JP, Weidinger S, Guttman-Yassky E, Phipatanakul W. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract 2021;9:1053ŌĆō65.



18. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis 2015;96:359ŌĆō61.

19. Riethmuller C, McAleer MA, Koppes SA, Abdayem R, Franz J, Haftek M, et al. Filaggrin breakdown products determine corneocyte conformation in patients with atopic dermatitis. J Allergy Clin Immunol 2015;136:1573ŌĆō80.



20. Lee HJ, Lee SH. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res 2014;6:276ŌĆō87.



21. Brunner PM, Guttman-Yassky E, Leung DYM. The immunology of atopic dermatitis and its reversibility with broadŌĆōspectrum and targeted therapies. J Allergy Clin Immunol 2017;139:65ŌĆō76.



22. Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy. Allergol Int 2020;69:204ŌĆō14.


23. Zhu TH, Zhu TR, Tran KA, Sivamani RK, Shi VY. Epithelial barrier dysfunctions in atopic dermatitis: a skinŌĆōgutŌĆō lung model linking microbiome alteration and immune dysregulation. Br J Dermatol 2018;179:570ŌĆō81.



24. Wang SH, Zuo YG. Thymic stromal lymphopoietin in cutaneous immune-mediated diseases. Front Immunol 2021;12:698522.



25. Brunner PM, Pavel AB, Khattri S, Leonard A, Malik K, Rose S, et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol 2019;143:142ŌĆō54.


26. Lou H, Lu J, Choi EB, Oh MH, Jeong M, Barmettler S, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial th2 cytokines and the GRP pathway. J Immunol 2017;198:2543ŌĆō55.




27. Sugaya M. The role of the 17-related cytokines in atopic dermatitis. Int J Mol Sci 2020;21:1314.


28. Furue M, Chiba T, Tsuji G, Ulzii D, Kido-Nakahara M, Nakahara T, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int 2017;66:398ŌĆō403.


29. Su├Īrez-Fari├▒as M, Dhingra N, Gittler J, Shemer A, Cardinale I, de Guzman Strong C, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol 2013;132:361ŌĆō70.



31. Furue M, Yamamura K, Kido-Nakahara M, Nakahara T, Fukui Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018;73:29ŌĆō36.



32. Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL, et al. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol 2019;143:26ŌĆō35.



33. Silverberg JI, Vakharia PP, Chopra R, Sacotte R, Patel N, Immaneni S, et al. Phenotypical differences of childhood-and adult-onset atopic dermatitis. J Allergy Clin Immunol Pract 2018;6:1306ŌĆō12.



34. Jeon YH, Ahn K, Kim J, Shin M, Hong SJ, Lee SY, et al. Clinical characteristics of atopic dermatitis in Korean school-aged children and adolescents according to onset age and severity. J Korean Med Sci 2022;37:e30.




35. Roduit C, Frei R, Depner M, Karvonen AM, Renz H, Braun-Fahrl├żnder C, et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr 2017;171:655ŌĆō62.


36. Bieber T, D'Erme AM, Akdis CA, Traidl-Hoffmann C, Lauener R, Sch├żppi G, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: where are we, and where should we go? J Allergy Clin Immunol 2017;139(4S): S58ŌĆō64.


37. Mold JE, Venkatasubrahmanyam S, Burt TD, Micha├½lsson J, Rivera JM, Galkina SA, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010;330:1695ŌĆō9.



38. Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol 2012;42:311ŌĆō9.



39. Brunner PM. Early immunologic changes during the onset of atopic dermatitis. Ann Allergy Asthma Immunol 2019;123:152ŌĆō7.


40. Czarnowicki T, He H, Canter T, Han J, Lefferdink R, Erickson T, et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J Allergy Clin Immunol 2020;145:215ŌĆō28.


41. Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016;138:1639ŌĆō51.

42. Kim J, Kim BE, Lee J, Han Y, Jun HY, Kim H, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol 2016;137:1282ŌĆō5.


43. Renert-Yuval Y, Del Duca E, Pavel AB, Fang M, Lefferdink R, Wu J, et al. The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J Allergy Clin Immunol 2021;148:148ŌĆō63.



45. Akdis CA, Akdis M. Immunological differences between intrinsic and extrinsic types of atopic dermatitis. Clin Exp Allergy 2003;33:1618ŌĆō21.



46. Park JH, Choi YL, Namkung JH, Kim WS, Lee JH, Park HJ, et al. Characteristics of extrinsic vs. intrinsic atopic dermatitis in infancy: correlations with laboratory variables. Br J Dermatol 2006;155:778ŌĆō83.



47. Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019;143:1ŌĆō11.


48. Gittler JK, Shemer A, Su├Īrez-Fari├▒as M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/ T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344ŌĆō54.



49. Tsoi LC, Rodriguez E, St├Člzl D, Wehkamp U, Sun J, Gerdes S, et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J Allergy Clin Immunol 2020;145:1406ŌĆō15.



50. Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 in’¼éammation in disease. Nat Rev Drug Discov 2016;15:35ŌĆō50.



51. Mack MR, Kim BS. The itch-scratch cycle: a neuroimmune perspective. Trends Immunol 2018;39:980ŌĆō91.



52. Silverberg JI, Kantor R. The Role of Interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clin 2017;35:327ŌĆō34.


53. Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020;156:44ŌĆō56.

54. Paller AS, Siegfried EC, Tha├¦i D, Wollenberg A, Cork MJ, Arkwright PD, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 2020;83:1282ŌĆō93.

55. Paller AS, Simpson EL, Siegfried EC, Cork MJ, Wollenberg A, Arkwright PD, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2022;400:908ŌĆō19.

56. Cork MJ, Tha├¦i D, Eichenfield LF, Arkwright PD, Hultsch T, Davis JD, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol 2020;182:85ŌĆō96.




57. ClinicalTrials.gov Identifier: NCT03050151. Study of dupilumab auto-injector device when used by patients with atopic dermatitis. https://clinicaltrials.gov/ct2/show/NCT03050151.
58. Blauvelt A, Guttman-Yassky E, Paller AS, Simpson EL, Cork MJ, Weisman J, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopic dermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol 2022;23:365ŌĆō83.




59. Akinlade B, Guttman-Yassky E, de Bruin-Weller M, Simpson EL, Blauvelt A, Cork MJ, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol 2019;181:459ŌĆō73.




60. Kamata M, Tada Y. A literature review of real-world effectiveness and safety of dupilumab for atopic dermatitis. JID Innov 2021;1:100042.



61. Gon alves F, Freitas E, Torres T. Selective IL-13 inhibitors for the treatment of atopic dermatitis. Drugs Context 2021;10:2021. ŌĆō1. -7.
63. Popovic B, Breed J, Rees DG, Gardener MJ, Vinall LMK, Kemp B, et al. Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of binding to IL-13r╬▒1 and IL-13r╬▒2. J Mol Biol 2013;425:1330ŌĆō9.


64. Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol 2021;184:437ŌĆō49.




65. Paller AS, Flohr C, Cork M, Bewley A, Blauvelt A, Hong HC, et al. Efficacy and Safety of Tralokinumab in Adolescents With Moderate to Severe Atopic Dermatitis: The Phase 3 ECZTRA 6 Randomized Clinical Trial. JAMA Dermatol 2023;159:596ŌĆō605.



66. Ultsch M, Bevers J, Nakamura G, Vandlen R, Kelley RF, Wu LC, et al. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol 2013;425:1330ŌĆō9.


67. Silverberg JI, Guttman-Yassky E, Tha├¦i D, Irvine AD, Gold LS, Blauvelt A, et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N Engl J Med 2023; 388:1080-91.69. Simpson EL, Gooderham M, Wollenberg A, Weidinger S, Armstrong A, Soung J, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol 2023;159:182ŌĆō91.


68. Simpson EL, Gooderham M, Wollenberg A, Weidinger S, Armstrong A, Soung J, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol 2023;159:182ŌĆō91.


69. Paller AS, Flohr C, Eichenfield LF, Irvine AD, Weisman J, Soung J, et al. Safety and Efficacy of Lebrikizumab in Adolescent Patients with Moderate-to-Severe Atopic Dermatitis: A 52-Week, Open-Label, Phase 3 Study. 2023;13:1517ŌĆō34.


70. Drucker AM, Ellis AG, Bohdanowicz M, Mashayekhi S, Yiu ZZN, Rochwerg B, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol 2020;156:659ŌĆō67.



71. Byeon JH, Yoon W, Ahn SH, Lee HS, Kim S, Yoo Y. Correlation of serum interleukin-31 with pruritus and blood eosinophil markers in children with atopic dermatitis. Allergy Asthma Proc 2020;1:59ŌĆō65.

72. Nobbe S, Dziunycz P, M├╝hleisen B, Bilsborough J, Dillon SR, French LE, et al. IL-31 expression by in’¼éammatory cells is preferentially elevated in atopic dermatitis. Acta Derm Venereol 2012;92:24ŌĆō8.

73. Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol 2018;27:327ŌĆō31.



74. Kabashima K, Matsumura T, Komazaki H, Kawashima M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med 2020;383:141ŌĆō50.


75. Sidbury R, Alpizar S, Laquer V, Dhawan S, Abramovits W, Loprete L, et al. Pharmacokinetics, safety, efficacy, and biomarker profiles during nemolizumab treatment of atopic dermatitis in adolescents. Dermatol Ther (Heidelb) 2022;12:631ŌĆō42.




76. Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, OŌĆÖShea JJ. JAK inhibition as a therapeutic strategy for immune and in’¼éammatory diseases. Nat Rev Drug Discov 2017;16:843ŌĆō62.



77. Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol 2021;148:927ŌĆō40.



78. Alexander M, Luo Y, Raimondi G, O'Shea JJ, Gadina M. Jakinibs of all trades: inhibiting cytokine signaling in immune-mediated pathologies. Pharmaceuticals (Basel) 2021;15:48.



79. Simpson EL, Lacour JP, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol 2020;183:242ŌĆō55.



80. Bieber T, Thyssen JP, Reich K, Simpson EL, Katoh N, Torrelo A, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol 2021;35:476ŌĆō85.



81. Torrelo A, Rewerska B, Galimberti M, Paller A, Yang CY, Prakash A, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in paediatric patients with moderate-to-severe atopic dermatitis with an inadequate response to topical corticosteroids: results from a phase III, randomized, double-blind, placebo-controlled study (BREEZE-AD PEDS). Br J Dermatol 2023;189:23ŌĆō32.



82. Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021;397:2151ŌĆō68.


83. Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021;397:2169ŌĆō81.


84. Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOT HERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019;393:2303ŌĆō11.


85. van Vollenhoven R, Takeuchi T, Pangan AL, Friedman A, Mohamed MEF, Chen S, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparatorŌĆōcontrolled trial. Arthritis Rheumatol 2020;72:1607ŌĆō20.




86. Simpson EL, Papp KA, Blauvelt A, Chu CY, Hong HC, Katoh N, et al. Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure Up 1 and Measure Up 2 Randomized Clinical Trials. JAMA Dermatol 2022;158:404ŌĆō413.



87. Silverberg JI, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, Costanzo A, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD Up study results. J Allergy Clin Immunol 2022;149:977ŌĆō87.e14.


88. Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020;396:255ŌĆō66.


89. Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2020;156:863ŌĆō73.



90. Cork MJ, McMichael A, Teng J, Valdez H, Rojo R, Chan G, et al. Impact of oral abrocitinib on signs, symptoms and quality of life among adolescents with moderate-to-severe atopic dermatitis: an analysis of patient-reported outcomes. J Eur Acad Dermatol Venereol 2022;36:422ŌĆō33.




91. Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: The JADE TEEN randomized clinical trial. JAMA Dermatol 2021;157:1165ŌĆō73.



92. Simpson EL, Silverberg JI, Nosbaum A, Winthrop KL, Guttman-Yassky E, Hoffmeister KM, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol 2021;22:693ŌĆō707.




-
METRICS

-
- 1 Crossref
- 0 Scopus
- 2,219 View
- 174 Download





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation

