< Previous Next >
Article Contents
| Clin Exp Pediatr > Volume 53(10); 2010 |
Abstract
The prevalence of pediatric obstructive sleep apnea syndrome (OSAS) is approximately 3% in children. Adenotonsillar hypertrophy is the most common cause of OSAS in children, and obesity, hypotonic neuromuscular diseases, and craniofacial anomalies are other major risk factors. Snoring is the most common presenting complaint in children with OSAS, but the clinical presentation varies according to age. Agitated sleep with frequent postural changes, excessive sweating, or abnormal sleep positions such as hyperextension of neck or abnormal prone position may suggest a sleep-disordered breathing. Night terror, sleepwalking, and enuresis are frequently associated, during slow-wave sleep, with sleep-disordered breathing. Excessive daytime sleepiness becomes apparent in older children, whereas hyperactivity or inattention is usually predominant in younger children. Morning headache and poor appetite may also be present. As the cortical arousal threshold is higher in children, arousals are not easily developed and their sleep architectures are usually more conserved than those of adults. Untreated OSAS in children may result in various problems such as cognitive deficits, attention deficit/hyperactivity disorder, poor academic achievement, and emotional instability. Mild pulmonary hypertension is not uncommon. Rarely, cardiovascular complications such as cor pulmonale, heart failure, and systemic hypertension may develop in untreated cases. Failure to thrive and delayed development are serious problems in younger children with OSAS. Diagnosis of pediatric OSAS should be based on snoring, relevant history of sleep disruption, findings of any narrow or collapsible portions of upper airway, and confirmed by polysomnography. Early diagnosis of pediatric OSAS is critical to prevent complications with appropriate interventions.
Obstructive sleep apnea syndrome (OSAS) is defined as a disorder of breathing during sleep characterized by prolonged partial airway obstruction and/or intermittent complete obstruction (obstructive apnea) that interrupts normal ventilation during sleep and normal sleep patterns1). OSAS is usually considered as an extreme of "sleep-disordered breathing" spectrum encompassing primary snoring, upper airway resistance syndrome, obstructive hypoventilation, and OSAS. Sleep-disordered breathing occurs during sleep and is exacerbated by sleep2). Primary snoring occurs when snoring is not associated with ventilatory abnormalities such as apnea, hypopnea, hypoxia, or hypercapnia3). Upper airway resistance syndrome is characterized by increasing negative intrathoracic pressure during inspiration without apparent apneic or hypopneic events, resulting in increased respiratory arousals leading to increased sleep fragmentation and daytime sleepiness. Obstructive hypoventilation is common in obese children, and diagnosed by snoring, reduced ventilatory drive with hypercapnea without apparent sleep apneas, or respiratory arousals4). Untreated sleep-disordered breathing in children has been reported to be related with various problems such as attention deficit/hyperactivity disorder, poor academic achievement, and behavioral problems. It may even cause more serious morbidities, such as growth failure, cor pulmonale, and systemic hypertension5). Moreover, recent studies suggest that primary snoring may not be as innocuous as previously thought, with learning, neurocognitive, and behavioral deficits being described in children who snore6, 7). This article reviews the prevalence, pathophysiology, and diagnosis of OSAS in children to improve the understanding of pediatric OSAS, and to promote early diagnosis and treatment to prevent serious complications.
The prevalence of habitual snoring extremely varies, from 3% to 35% of children under 13 years of age, due to the different definition of habitual snoring and epidemiologic methodologies8,9); however, it has been mostly reported in 8-12% of children from 2 to 8 years of age10-13). OSAS has been estimated to affect about 2-3.5% of children8, 13,14). OSAS prevalence has 2 peak periods. The first peak occurs in children from 2 to 8 years of age, with the presence of enlarged adenoid and/or tonsils. A second peak arises during adolescence in relation with weight gain. Although gender differences have not been observed in the prevalence of OSAS among prepubertal children, some adolescent boys has been more affected than girls in a few reports12). In adults, most studies have estimated a sex-specific prevalence of a 2- to 3-fold greater risk for men than women15). In an epidemiologic study for Korean adolescents from 15 to 18 years of age, the prevalence of snoring and OSAS were 11.2% and 0.9%, respectively. Snoring was more common in boys (12.4% in boys vs. 8.5% in girls)11). The prevalence of sleep-disordered breathing in Korean children has not been reported.
The upper airway consists of the nose, pharynx, larynx, and extrathoracic trachea, which have important physiologic functions such as respiration, swallowing, speech and vibration, and local immunity. The pharynx is a collapsible segment that performs these functions under the balanced control of dilator and constrictor muscles. During the waking state, upper airway collapse is prevented by keeping an increased pharyngeal neuromuscular tone16). This mechanism, however, is attenuated during sleep, predisposing the upper airway to collapse17).
The main risk factors for OSAS in adults are obesity and male sex, which are related to the propensity for repetitive upper airway collapse18). In younger children, the major risk factor for the development of OSAS is adenotonsillar hypertrophy19). Adenoid and tonsils grow progressively during childhood20-22), whereas the skeletal boundaries of the upper airway slowly expand. Between the ages of 3 and 8 years, the tonsils and adenoid are largest in relation to the underlying upper airway size, which makes a narrow upper airway. This size disparity coincides with the peak incidence of pediatric OSAS5). Neuromuscular factors for upper airway patency are affected by central ventilatory drive, arousal responses to respiratory occlusion (arousal threshold), and upper airway reflexes23, 24). It is suggested that central ventilatory drive is increased during childhood and then declines gradually with age25, 26). This increased central ventilatory drive during sleep accounts for the increased upper airway reflexes and tone in children, resulting in less collapsibility than in adults. Thus, children have a specific breathing pattern of obstructive hypoventilation rather than discrete, cyclic obstructive pattern that is commonly seen in adult sleep-disordered breathing27). Children also have a higher arousal threshold than adults; the younger the child, the higher the threshold of arousal28). Children are, therefore, less likely to arouse in response to upper airway obstruction than adults, accounting for the preservation of sleep architecture29).
In obese children, excessive deposition of fat tissue within the muscles and tissue surrounding the upper airway leads to reduced airway size and increased airway resistance22). Reduced lung volumes and decreased central ventilatory drive in obese children also contribute to compromised upper airway patency30). Nowadays, obesity is a major cause of pediatric OSAS in the western countries due to the dramatic increase of the prevalence of obesity in children. A large epidemiologic study showed that obesity is the most significant risk factor for developing OSAS in children between 2 and 18 years of age, with an odds ratio of 4.531). Recently, it is suggested that pediatric OSAS with obesity would be classified as "pediatric OSAS type 2" since it has similar clinical features with adult OSAS32).
Nasal mucosal edema induced by allergic rhinitis, which increase nasal resistance, may also exacerbate or induce sleep-breathing disorder in children and adolescents1). Craniofacial anomalies such as midfacial hypoplasia, small nasopharynx, and/or micrognathia in Pierre Robin sequence, Apert syndrome, and Marfan syndrome are also important risk factors for developing OSAS19). OSAS presents in 30% to 60% of individuals with Down syndrome, related with decreased nasopharyngeal surface area, ventilatory volume, and hypotonia19, 33, 34). Hypothyroidism, a frequent complication of Down syndrome, is another risk factor, which leads to hypotonia. Neuromuscular disease such as progressive muscular dystrophy is related with reduced upper airway muscle tone, resulting in upper airway collapse.
Genetic risk factors have been identified in the development of OSAS35). Asian adults tend to have more severe OSAS for an even lesser degree of obesity than Caucasians36). The short skull base has been noted as an ethnic risk factor in Asians. Asian children also have more severe OSAS than Caucasians, although prevalence is relatively lower37). In African Americans, thick soft tissue dimensions such as tongue mass and oral mucosa are risk factors for OSAS. African American children have been shown to have 4- to 6-fold higher risks than white children, independent of other factors such as obesity, premature birth, and maternal smoking38).
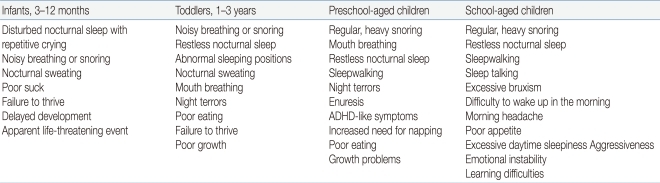
A wide range of symptoms and signs are associated with OSAS in children depending on their developmental stages (Table 1)39). Snoring is the most common presenting complaint of children and adolescents with OSAS. Parents may describe chest wall retractions, paradoxical breathing, and sometimes pauses in breathing. Restless sleep with frequent changes of body position is also well described among children with OSAS, although many parents consider it a normal behavior during sleep in childhood. Excessive sweating during sleep may be related with labored breathing. Abnormal sleep positions such as hyperextension of the neck in infants and abnormal prone position may indicate the possibility of having sleep-disordered breathing. Night terror and sleepwalking are frequently accompanied by sleep-disordered breathing during slow-wave sleep in children and adolescents with a positive family history of parasomnias40). Children with OSAS are high risk for enuresis, which may resolve when the OSAS is adequately treated41). Excessive daytime sleepiness is a typical symptom in adolescents with OSAS, whereas hyperactivity or inattention is predominant in preadolescent children with sleep-disordered breathing. Morning headaches and poor appetite may present in OSAS, which may be due to carbon dioxide retention, sleep fragmentation, or gastroesophageal reflux.
Untreated pediatric OSAS can result in serious morbidity in neurobehavioral, cardiovascular, and somatic growth and development. Many studies have shown evidence for clear associations between OSAS and hyperactivity, attention deficit, and other behavioral problems such as social withdrawal or aggression42-45). The prevalence of attention deficit/hyperactivity (ADHD) in the school-age population is 8-10%46), while 20-30% of children with snoring and/or OSAS have clinically significant problems with inattention and hyperactivity47). This ADHD-like features in children with OSAS may result from repeated sleep disruptions and intermittent hypoxic episodes that affect prefrontal executive function such as working memory, behavioral control, analysis, organization, and self-regulation of motivation42, 48). The prefrontal cortex is also believed to be responsible for the regulation of arousal, sleep, affect, and attention, as well as executive functions49). Behavioral deficit and executive dysfunction in children with OSAS have been also shown to have negative impact on learning and school performance50, 51). A number of studies have suggested that therapeutic intervention of OSAS such as adenoidectomy and/or tonsillectomy have a significant improvement of not only the abnormal behaviors such as hyperactivity, inattention, and aggression but also cognition and school performance47, 52-55).
Cor pulmonale with heart failure and pulmonary hypertension were not an uncommon mode of presentation in children with a history of OSAS56, 57); however, they are now rare due to early detection and treatment of OSAS. Although obvious right heart failure now occurs less often, asymptomatic pulmonary hypertension may be common58). Systemic hypertension can occur57). The pathophysiology of cardiovascular complications is as follows: intermittent upper airway obstruction during sleep in OSAS patients that have induced an exaggeration of continuous negative intrathoracic pressure swings, leading to a second series of sustained alterations of blood pressure and endothelial function, and eventually changes in cardiac structure and function occurs probably via oxidative stress and increased sympathetic tone59-65). Increased proinflammatory cytokine such as interleukin-6 or C-reactive protein have been noted in children with OSAS, which may be linked to endothelial dysfunction and atherogensis66). It is suggested that systemic inflammation is a consequence of OSAS even in the absence of obesity, and is reversible after treatment of OSAS in most patients67). Failure to thrive is not a common consequence of OSAS in children; however, growth spurt after adenotonsillectomy is commonly reported68, 69). Growth failure is possibly related to a combination of anorexia and decreased oral intake, increased energy consumption from increased work of breathing, and alternations in nocturnal growth hormone secretion patterns70, 71). Many studies have been shown that catch-up growth is related with increased secretion of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 3 (IGFBP-3) after adenotonsillectomy, both of which are highly correlated with diurnal growth hormone secretion and reflect mean daily growth hormone levels70, 72-74).
Diagnosis of OSAS in children is made on the basis of sleep history, physical examination, and polysomnographic findings (Table 2)75). OSAS should be suspected on parental complaints about their children's sleep. Frequent napping or excessive sleepiness in the classroom is a major clue to sleep problems in older children. Clinicians can use a sleep log, sleep diary, or sleep questionnaire to efficiently identify the traits of a patient's sleep. Although a thorough history of sleep is very important in the diagnosis of sleep disorders, studies have shown that OSAS cannot be differentiated from primary snoring by history alone1). A comprehensive physical examination of the upper airway from the nose to the oropharynx can help find any anatomical narrowing; that is, a deviated nasal septum, enlarged inferior turbinate, overcrowding of teeth, enlargement of adenoid with/without tonsillar hypertrophy, or the presence of a high and narrow hard palate. Mallampati score is helpful in evaluating the patency of airway related to tonsil size, particularly for older and obese children76) (Fig. 1). An adenoid face with mouth breathing at the waking state is an important clue in detecting sleep-disordered breathing. Inspection of the lateral facial profile is helpful to evaluate for retrognathia, micrognathia, or midfacial hypoplasia.
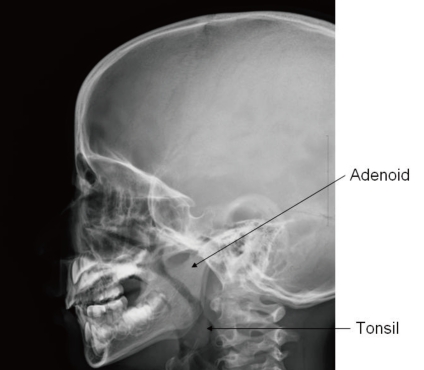
In advanced cases of OSAS, a loud second pulmonary heart sound may manifest as an evidence of pulmonary hypertension63). Assessment for growth and development should not be omitted because growth impairment and delayed development are frequently associated in children with OSAS77). Paranasal sinus with neck lateral view in X-ray is a simple but very useful method for the detection of sinusitis or adenoid hypertrophy (Fig. 2). Endoscopy performed under sedation is useful in localizing the region of maximal airway restriction. This technique is reserved for children with complicated airway structure and altered collapsibility such as congenital craniofacial anomaly78).
The American Academy of Pediatrics (AAP) suggested a clinical guideline for childhood OSAS in 200256) (Table 3). The AAP recommended polysomnography (PSG) as the only gold standard method for the diagnosis of pediatric OSAS. While they accepted the use of audiovisual taping and pulse oximetry recording as screening studies for OSAS, PSG should be followed in clinically suspected children if these screening tests do not support OSAS. A guideline for PSG in children is recommended by the American Academy of Sleep Medicine (AASM) in 2007 (Table 4)79). Supervision by a trained technician is required throughout the study, with additional record keeping of unusual events or behaviors during the night. An extended full EEG channel is useful for differentiation of nocturnal seizure disorders (e.g., nocturnal frontal lobe epilepsy) from parasomnias such as sleepwalking. Endtidal CO2 measurement can also help assess gas exchange. This is particularly important in children with obesity or neuromuscular diseases, who have a higher risk for hypoventilation78). Balloon esophageal manometry, which can detect upper airway resistance by measuring the intrathoracic negative pressure, is now rarely used in children due to intolerability. New techniques such as pulse transit time or cyclic alternating pattern on EEG are becoming more convenient tools to detect arousals during PSG. These methods are more sensitive and may predict neurocognitive and/or cardiovascular outcomes in children with OSAS80-82).
The PSG parameters that are mostly used in evaluating for OSAS are the apnea-hypopnea index (AHI), apnea index (AI), respiratory event-related arousals (RERAs), and respiratory disturbance index (RDI). AHI indicates the number of apneic and hypopneic events per hour of sleep. Apnea is defined as a complete interruption of airflow lasts at least 2 breath periods in children or 10 seconds in adult, whereas hypopnea is defined as a ≥50% reduction in airflow with an arousal, awakening, or ≥3% desaturation for same durations by using a nasal cannula-pressure transducer79) (Fig. 3). AI indicates the number of obstructive and/or central apneic events per hour of sleep. RERA is defined as a sequence of breath lasts at least 2 breath periods, which does not meet the criteria for apnea or hypopnea but is accompanied by increasing respiratory effort, leading to an arousal from sleep. The AHI and RERA may be reported together as the RDI. The diagnosis of OSAS in children is defined as AHI index >1, according to the criteria of the AASM75), although different criteria for childhood OSAS, such as AI >1 or RDI >1.5, have been suggested in several studies39, 83). Sleep-related hypoventilation may be scored when >25% of the total sleep time is spent with a CO2 >50 mm Hg, measured by end-tidal CO2 (PETco2) and/or transcutaneous CO2 (Ptco2) sensors79, 84) (Table 5). It should be noted that common PSG parameters such as AI, AHI, RDI, and the nadir of oxygen saturation (SpO2) are helpful in evaluating the severity of OSAS, but they may not completely identify sleep-disordered breathing in children since PSG has not been well standardized in its performance or interpretation56). Other important clinical parameters such as paradoxical respiration, duration of abnormal sleep position, and frequency of body position change may be quantified and included as criteria for the diagnosis of childhood OSAS85). Therefore, a thorough validity study for current PSG parameters with the development of new diagnostic criteria for pediatric OSAS is mandatory.
Pediatric OSAS has been commonly overlooked and underdiagnosed by both parents and clinician until now. Clinicians should be familiar with the manifestations of OSAS because children have various clinical symptoms and signs according to their developmental stages. OSAS in children are also different from OSAS in adults, in particular with respect to pathophysiology, gender distribution, and nocturnal and daytime symptoms. Children with OSAS but were not treated may have serious morbidities such as neurobehavioral, cardiovascular, and impairment of growth and development, most of which are reversible by early detection and treatment. Although clinical parameters in the pediatric PSG field are still evolving, PSG is a good diagnostic tool for the diagnosis of pediatric OSAS.
References
1. American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996;153:866–878.


3. Ali NJ, Pitson D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child 1994;71:74–76.



4. Fiorino EK, Brooks LJ. Obesity and respiratory diseases in childhood. Clin Chest Med 2009;30:601–608. x


5. Benninger M, Walner D. Obstructive sleep-disordered breathing in children. Clin Cornerstone 2007;9(Suppl 1): S6–S12.


6. Blunden S, Lushington K, Kennedy D, Martin J, Dawson D. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol 2000;22:554–568.


7. Urschitz MS, Guenther A, Eggebrecht E, Wolff J, Urschitz-Duprat PM, Schlaud M, et al. Snoring, intermittent hypoxia and academic performance in primary school children. Am J Respir Crit Care Med 2003;168:464–468.


8. Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest 1995;107:963–966.


9. Castronovo V, Zucconi M, Nosetti L, Marazzini C, Hensley M, Veglia F, et al. Prevalence of habitual snoring and sleep-disordered breathing in preschool-aged children in an Italian community. J Pediatr 2003;142:377–382.


10. Teculescu DB, Caillier I, Perrin P, Rebstock E, Rauch A. Snoring in French preschool children. Pediatr Pulmonol 1992;13:239–244.


11. Shin C, Joo S, Kim J, Kim T. Prevalence and correlates of habitual snoring in high school students. Chest 2003;124:1709–1715.


12. Goodwin JL, Babar SI, Kaemingk KL, Rosen GM, Morgan WJ, Sherrill DL, et al. Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children's Assessment of Sleep Apnea Study. Chest 2003;124:196–203.


13. Schlaud M, Urschitz MS, Urschitz-Duprat PM, Poets CF. The German study on sleep-disordered breathing in primary school children: epidemiological approach, representativeness of study sample, and preliminary screening results. Paediatr Perinat Epidemiol 2004;18:431–440.


14. Shine NP, Coates HL, Lannigan FJ. Obstructive sleep apnea, morbid obesity, and adenotonsillar surgery: a review of the literature. Int J Pediatr Otorhinolaryngol 2005;69:1475–1482.


15. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217–1239.


16. Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 1992;89:1571–1579.



17. Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med 1996;153:1880–1887.


18. Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994;149:722–726.


19. Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep 2004;27:997–1019.


20. Vogler RC, Ii FJ, Pilgram TK. Age-specific size of the normal adenoid pad on magnetic resonance imaging. Clin Otolaryngol Allied Sci 2000;25:392–395.


21. Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol 1979;133:401–404.


23. Glomb WB, Marcus CL, Keens TG, Ward SL. Hypercapnic and hypoxic ventilatory and cardiac responses in school-aged siblings of sudden infant death syndrome victims. J Pediatr 1992;121:391–397.


24. Isono S. Developmental changes of pharyngeal airway patency: implications for pediatric anesthesia. Paediatr Anaesth 2006;16:109–122.


25. Nishimura M, Yamamoto M, Yoshioka A, Akiyama Y, Kishi F, Kawakami Y. Longitudinal analyses of respiratory chemosensitivity in normal subjects. Am Rev Respir Dis 1991;143:1278–1281.


26. Marcus CL, Glomb WB, Basinski DJ, Davidson SL, Keens TG. Developmental pattern of hypercapnic and hypoxic ventilatory responses from childhood to adulthood. J Appl Physiol 1994;76:314–320.


27. Isono S, Tanaka A, Ishikawa T, Nishino T. Developmental changes in collapsibility of the passive pharynx during infancy. Am J Respir Crit Care Med 2000;162:832–836.


28. Busby KA, Mercier L, Pivik RT. Ontogenetic variations in auditory arousal threshold during sleep. Psychophysiology 1994;31:182–188.


29. Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med 2000;162:682–686.


30. Mallory GB Jr, Fiser DH, Jackson R. Sleep-associated breathing disorders in morbidly obese children and adolescents. J Pediatr 1989;115:892–897.


31. Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med 1999;159:1527–1532.


32. Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: One or two distinct disease entities? Sleep Med Clin 2007;2:433–444.



33. Bacon WH, Krieger J, Turlot JC, Stierle JL. Craniofacial characteristics in patients with obstructive sleep apneas syndrome. Cleft Palate J 1988;25:374–378.

34. Redline S, Leitner J, Arnold J, Tishler PV, Altose MD. Ventilatory-control abnormalities in familial sleep apnea. Am J Respir Crit Care Med 1997;156:155–160.


35. Gaultier C, Guilleminault C. Genetics, control of breathing, and sleep-disordered breathing: a review. Sleep Med 2001;2:281–295.


36. Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev 2005;9:419–436.


37. Anuntaseree W, Rookkapan K, Kuasirikul S, Thongsuksai P. Snoring and obstructive sleep apnea in Thai school-age children: prevalence and predisposing factors. Pediatr Pulmonol 2001;32:222–227.


38. Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr 2003;142:383–389.


39. Guilleminault C, Lee JH, Chan A. Pediatric obstructive sleep apnea syndrome. Arch Pediatr Adolesc Med 2005;159:775–785.


40. Guilleminault C, Palombini L, Pelayo R, Chervin RD. Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics 2003;111:e17–e25.


42. Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep 1997;20:1185–1192.


43. Owens J, Spirito A, Marcotte A, McGuinn M, Berkelhammer L. Neuropsychological and behavioral correlates of obstructive sleep apnea syndrome in children: A preliminary study. Sleep Breath 2000;4:67–78.


44. Chervin RD, Archbold KH, Dillon JE, Panahi P, Pituch KJ, Dahl RE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics 2002;109:449–456.


45. Gottlieb DJ, Vezina RM, Chase C, Lesko SM, Heeren TC, Weese-Mayer DE, et al. Symptoms of sleep-disordered breathing in 5-year-old children are associated with sleepiness and problem behaviors. Pediatrics 2003;112:870–877.


46. American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158–1170.


47. Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur J Pediatr 1996;155:56–62.


48. Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 2002;11:1–16.

49. Dahl RE. The impact of inadequate sleep on children's daytime cognitive function. Semin Pediatr Neurol 1996;3:44–50.


50. Urschitz MS, Eitner S, Guenther A, Eggebrecht E, Wolff J, Urschitz-Duprat PM, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics 2004;114:1041–1048.


51. Montgomery-Downs HE, Crabtree VM, Gozal D. Cognition, sleep and respiration in at-risk children treated for obstructive sleep apnoea. Eur Respir J 2005;25:336–342.


52. Chervin RD, Ruzicka DL, Giordani BJ, Weatherly RA, Dillon JE, Hodges EK, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 2006;117:e769–e778.



53. Friedman BC, Hendeles-Amitai A, Kozminsky E, Leiberman A, Friger M, Tarasiuk A, et al. Adenotonsillectomy improves neurocognitive function in children with obstructive sleep apnea syndrome. Sleep 2003;26:999–1005.


54. Avior G, Fishman G, Leor A, Sivan Y, Kaysar N, Derowe A. The effect of tonsillectomy and adenoidectomy on inattention and impulsivity as measured by the Test of Variables of Attention (TOVA) in children with obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2004;131:367–371.


55. Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 1998;102:616–620.


56. American Academy of Pediatrics. Clinical practice guideline: Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704–712.


57. Guilleminault C, Eldridge FL, Simmons FB, Dement WC. Sleep apnea in eight children. Pediatrics 1976;58:23–30.



58. Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment. Pediatr Pulmonol 1988;4:139–143.


59. Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea-induced hypoxemia during sleep. Chest 2001;119:1085–1091.


60. Kwok KL, Ng DK, Cheung YF. BP and arterial distensibility in children with primary snoring. Chest 2003;123:1561–1566.


61. Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521–530.


62. Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics 2004;113:e564–e569.


63. Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med 1998;157:1098–1103.


64. Sofer S, Weinhouse E, Tal A, Wanderman KL, Margulis G, Leiberman A, et al. Cor pulmonale due to adenoidal or tonsillar hypertrophy or both in children. Noninvasive diagnosis and follow-up. Chest 1988;93:119–122.


65. Amin RS, Kimball TR, Kalra M, Jeffries JL, Carroll JL, Bean JA, et al. Left ventricular function in children with sleep-disordered breathing. Am J Cardiol 2005;95:801–804.


66. Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med 2008;9:254–259.


67. Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation 2007;116:2307–2314.


68. Bar A, Tarasiuk A, Segev Y, Phillip M, Tal A. The effect of adenotonsillectomy on serum insulin-like growth factor-I and growth in children with obstructive sleep apnea syndrome. J Pediatr 1999;135:76–80.


69. Bonuck KA, Freeman K, Henderson J. Growth and growth biomarker changes after adenotonsillectomy: systematic review and meta-analysis. Arch Dis Child 2009;94:83–91.


70. Kang JM, Auo HJ, Yoo YH, Cho JH, Kim BG. Changes in serum levels of IGF-1 and in growth following adenotonsillectomy in children. Int J Pediatr Otorhinolaryngol 2008;72:1065–1069.


71. Bonuck K, Parikh S, Bassila M. Growth failure and sleep disordered breathing: a review of the literature. Int J Pediatr Otorhinolaryngol 2006;70:769–778.


72. Lindgren BF, Segovia B, Lassarre C, Binoux M, Gourmelen M. Growth retardation in constitutionally short children is related both to low serum levels of insulin-like growth factor-I and to its reduced bioavailability. Growth Regul 1996;6:158–164.

73. Furlanetto RW. Insulin-like growth factor measurements in the evaluation of growth hormone secretion. Horm Res 1990;33(Suppl 4): 25–30.


74. Kiris M, Muderris T, Celebi S, Cankaya H, Bercin S. Changes in serum IGF-1 and IGFBP-3 levels and growth in children following adenoidectomy, tonsillectomy or adenotonsillectomy. Int J Pediatr Otorhinolaryngol 2010;74:528–531.


75. International Classification of Sleep Disorders-ICSD. 2005;2nd ed. Westchester, IL: American Academy of Sleep Medicine.
76. Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985;32:429–434.


77. Marcus CL, Carroll JL, Koerner CB, Hamer A, Lutz J, Loughlin GM. Determinants of growth in children with the obstructive sleep apnea syndrome. J Pediatr 1994;125:556–562.


78. Brooks LJ. Diagnosis and evaluation of obstructive sleep apnoea in children. Ann Acad Med Singapore 2008;37:701–705.


79. Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 2007;Westchester, IL: American Academy of Sleep Medicine.
80. Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res 2003;53:580–588.


81. Miano S, Rizzoli A, Evangelisti M, Bruni O, Ferri R, Pagani J, et al. NREM sleep instability changes following rapid maxillary expansion in children with obstructive apnea sleep syndrome. Sleep Med 2009;10:471–478.


82. Lopes MC, Guilleminault C. Chronic snoring and sleep in children: a demonstration of sleep disruption. Pediatrics 2006;118:e741–e746.


83. Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis 1992;146:1235–1239.


84. Mindell JA, Owens JA. A clinical guide to pediatric sleep: Diagnosis and management of sleep. 2010;2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins.
Fig. 1
Mallampati score. Class 1: full visibility of tonsils, uvula, and soft palate. Class 2: visibility of hard and soft palate, upper portion of tonsils, and uvula. Class 3: soft and hard palate and base of the uvula are visible. Class 4: only hard palate is visible. Higher scores are correlated with having OSAS.

Fig. 3
Obstructive hypopnea (60-second PSG epoch) in REM sleep. The event (arrow) was initiated by diminished nasal pressure airflow (TPAF) accompanied by paradoxical respiration leading to arousal. This respiratory event was associated with a ≥50% decrease in the amplitude of the nasal pressure signal. Ocular movement is seen on the electrooculogram (LOC-M2, ROC-M1).

Table 2
Diagnostic Criteria of Pediatric Obstructive Sleep Apnea by the AASM*

*American Academy of Sleep Medicine75)
Table 3
Clinical Guideline for Childhood OSAS*

*The American Academy of Pediatrics (AAP) have developed a clinical guideline for childhood OSAS in 200256).
Table 4
Polysomnographic Variables recommended by the AASM*
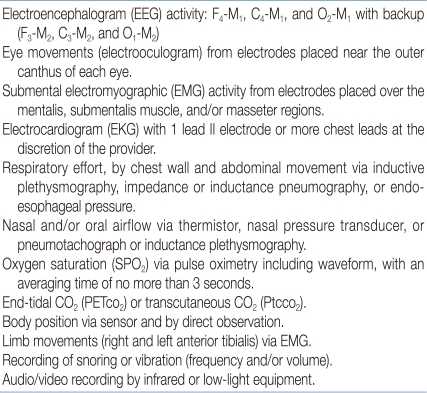
*AASM: American Academy of Sleep Medicine79)
F: frontal electrode; M: mastoid electrode; C: central electrode; O: occipital electrode.
Table 5
Polysomnographic Criteria* for Pediatric OSAS

This pediatric PSG criteria is cited from a clinical guide to pediatric sleep, with modification of the cutoff value84).



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


