< Previous Next >
Article Contents
| Korean J Pediatr > Volume 55(3); 2012 |
Abstract
Human astrovirus (HAstV) is a major cause of acute diarrhea among children, resulting in outbreaks of diarrhea and occasionally hospitalization. Improved surveillance and application of sensitive molecular diagnostics have further defined the impact of HAstV infections in children. These studies have shown that HAstV infections are clinically milder (diarrhea, vomiting, fever) than infections with other enteric agents. Among the 8 serotypes of HAstV identified, serotype 1 is the predominant strain worldwide. In addition to serotype 1, the detection rate of HAstV types 2 to 8 has increased by using newly developed assays. HAstV is less common compared with other major gastroenteritis viruses, including norovirus and rotavirus; however, it is a potentially important viral etiological agent with a significant role in acute gastroenteritis. A better understanding of the molecular epidemiology and characteristics of HAstV strains may be valuable to develop specific prevention strategies.
Human astrovirus (HAstV), along with rotavirus and calicivirus, is recognized as a common cause of infantile acute gastroenteritis1). HAstV-related diseases in humans have been determined through a combination of epidemiological surveys and clinical observations. Eight serotypes of HAstV have been identified to date, with HAstV-1 being most commonly detected2-5). HAstV infects a variety of demographics, including elderly4), immunocompromised6,7), healthy, and immunocompetent adults8-10). However, the most commonly affected group is children under the age of 2 years. Transmission in children occurs usually from person to person. Symptoms manifest within 2 to 3 days post-infection and last for approximately the same amount of time. HAstV infection is associated primarily with diarrhea, although vomiting, abdominal pain, headache, and mild dehydration are occasionally observed as well11). HAstV-induced diarrhea is generally not severe enough to require hospitalization and resolves spontaneously2). HAstV infections have been reported in 2 to 16% of children hospitalized with diarrhea and in 5 to 17% of children with diarrhea in community studies12-16). Although HAstV was detected less frequently than rotavirus or norovirus in surveillance studies, HAstV still requires close attention from a public health viewpoint because of large outbreaks in adults8,10) and frequent outbreaks in schools, pediatric hospitals, and child-care and aged-care centers1,17-19).
This review focuses on recent papers on the epidemiology and clinical characteristics of HAstV serotypes as infectious agents in diarrhea among hospitalized children, in children from the community, and in outbreaks. Control measures are discussed and future immunization strategies are considered.
AstVs are single-stranded, positive-sense RNA viruses that are closely related to other small round-structured viruses, such as calicivirus and picornavirus. The Astroviridae family was divided into 2 genera by the International Committee on Taxonomy of Viruses, i.e., Mamastrovirus and Avastrovirus (Fig. 1). Mamastrovirus includes HAstVs that are named according to the order of discovery, types 1 to 8, and animal AstVs infecting kittens, piglets, puppies, cattle (types 1 and 2), sheep, deer, and mink kittens. Avastrovirus includes duck AstV, turkey AstVs (types 1 and 2), and avian nephritis virus of chickens (types 1 and 2). Members of this genus infect avian species causing a variety of manifestations, including enteritis, hepatitis, nephritis, and fatal immunosuppression.
The genome of AstVs is 6844-7355 base pairs in length and includes 3 open reading frames (ORFs), ORF1a, ORF1b, and ORF2. AstV non-structural polyproteins are encoded in the first 2 ORFs linked by a ribosomal frame-shifting event. ORF1a encodes a nonstructural polyprotein, nsP1a, which displays a 3C-like serine protease motif20). NsP1a is post-translationally cleaved into functional small peptides. Cleavage sites were identified by mapping of these small peptides. Mutations in the 3C-like serine protease active site resulted in undetectable levels of some, but not all, proteolytic cleavage products, supporting the partial involvement of the AstV 3C-like serine protease in the autocatalytic processing of nsP1a21). ORF1b encodes the viral RNA-dependent RNA polymerase and ORF2 encodes the precursor capsid (outer coat) of the virus. In the maturation process, the full-length precursor capsid protein is post-translationally modified and can assemble into viral particles. However, proteolytic cleavage of the precursor capsid protein is required for an AstV particle to be infectious. Trypsin treatment of these particles results in the generation of 2 to 3 small proteins, which correlate with the greatest infectivity22,23). The genetic sequence of the capsid region (ORF2) is used to construct the evolutionary relatedness of animal AstVs and HAstVs using phylogenetic tools. Phylogenetic analysis demonstrated that human viruses clustered together and were distinct from non-human viruses, which argues for a common evolutionary origin and against ongoing animal-to-human transmissions24). HAstVs appear to have crossed species (possibly from felines) a long time ago and continued to diverge and evolve since that time. In contrast, other studies suggest interspecies transmissions, involving humans, cats, and pigs, relatively recently in the evolutionary history of AstVs. There is also evidence of infection of both turkeys and chickens by avian nephritis virus, which questions the theory of "species-specific" infections25).
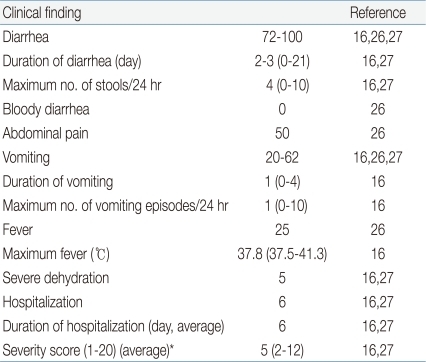
The clinical features of HAstV infections may also depend on several factors. Several studies highlighted frequent co-infections of HAstV with rotavirus and calicivirus (13 to 65%)16). HAstV-associated diarrhea has been characterized by a median duration of 3 days (range, 1 to 21 days), median of 4 stools (range, 1 to 10 stools) during the first 24 hours, vomiting (20 to 62%), and fever (7 to 25%). HAstV-associated diarrhea was less severe than rotavirus-induced diarrhea, as measured by the number of stools and duration of diarrhea. Fever and duration of vomiting and diarrhea were significantly more frequent during isolated rotavirus infections than in isolated AstV infections16,26,27). Only 0 to 3% of cases of HAstV gastroenteritis resulted in dehydration, and no repeat symptomatic infections were observed. Very few AstV-infected children (3%) were hospitalized26,27). In addition, bloody diarrhea and vomiting are statistically less common in viral than in bacterial gastroenteritis (Table 1)16). The overall severity of HAstV infections was the lowest among the enteric agents examined26,28). The severity of HAstV infection by genotypes is not fully elucidated.
The results of surveillance studies for HAstVs depend upon the method utilized. In early surveys using electron microscopy, HAstV appeared to be a rare cause of gastroenteritis, being found in less than 1% of children with diarrhea, usually in small outbreaks of the disease, and primarily during the winter season. The development and use of monoclonal antibodies and enzyme immunoassays to detect HAstV led to reports of a higher prevalence (2.5 to 9%) of HAstV infection among patients hospitalized with diarrhea. The cloning and sequencing of AstVs have led to the development of more sensitive assays to detect the viruses by using reverse transcription-polymerase chain reaction (RT-PCR). Application of RT-PCR for the detection of HAstVs in children in day care centers showed a marked increase in the detected prevalence of HAstV-associated diarrhea, the rate of asymptomatic infections, and the duration of shedding of the virus among those infected, when compared with studies that used other methods16,26). Both immunoassays and molecular-based assays to detect HAstV serotypes indicate that serotype 1 is most common worldwide, although the predominant serotypes may vary by region and season.
Several recent studies investigated viral etiologies for infectious diarrhea among children who were hospitalized or in the communities in different parts of world (China, Korea, Japan, and France)26,29-31).
The study in China29) was conducted between 1998 and 2005 among children 5 years of age hospitalized for acute gastroenteritis in 7 provinces of China. Stool samples from patients with diarrhea were tested for HAstV by enzyme immunoassay (EIA) or RT-PCR. The detection rate of HAstV infection was 5.5% (91/1,668) and diarrhea cases caused by HAstV infection could be found in any season of the year but mainly occurred during the cold season from October to January.
In the study conducted in Korea recently30), the frequency of HAstV infection was determined by EIA in 160,027 patients with diarrhea between 2002 and 2007. A total of 2,057 samples (1.3%) (detection rate per year, 0.6 to 2.4%) were identified as positive for HAstV. One study showed that HAstV appears to cause diarrhea with similar frequencies in children and adults32). Nosocomial HAstV infection also is a concern. A study in Stanford, California, demonstrated that 10% of inpatient children with diarrhea had HAstV infection and half of these infections were nosocomial33).
The age distribution of HAstV infections may vary depending on the clinical settings, geographic location, and the age distribution of the population analyzed. In a hospital-based study in China, the age distribution of HAstV infection was 7.4% among infants aged 9 to 11 months, followed by 6.1% in 12 to 17-month-old children, 5.6% among children aged 6 to 8 months, and 5.6% in 0 to 2-month-old infants. Over 95% of HAstV infections occurred in children of less than 2 years of age29).
The seasonality of HAstV infections is controversial and seems to vary by geographic region. Studies demonstrated that 34 to 60% of cases of diarrhea among children in the United States, France, and Finland have a viral causative agent appearing in late winter and spring34). In Korea, the majority of HAstV infections among hospitalized children occurred in winter, simultaneously with the highest number of infections with rotavirus36). In Vietnam, which has a tropical climate, HAstVs infections were found during 2 distinct periods, i.e., from March to May, which is the end of the dry season and beginning of the rainy season, and during the rainy season, which lasts from August to November. The detected HAstVs were identified in the rainy season37).
Early studies reported that HAstV-1 is also the predominant strain in Egypt, Italy, France, China, and Spain16,30). The detection rate of HAstV types 2 to 8 has increased by using newly developed assays. This phenomenon either may be due to the increased sensitivity of the assays utilized or indicates that HAstV-8 may be a newly emerging type.
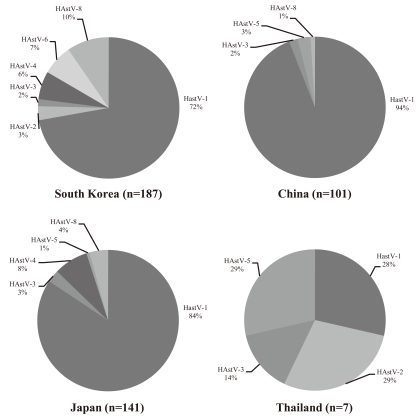
In Korea, China, and Japan, HAstV-1 accounted for over 70% of all HAstV infections analyzed, whereas in Thailand, the relative frequency of HAstV-1 was 28% (Fig. 2). In Korea, the overall distribution of the genotypes of the 187 HAstV strains detected in clinical specimens and characterized was as follows: HAstV-1, 72.19%; HAstV-8, 9.63%; HAstV-6, 6.95%; HAstV-4, 6.42%; HAstV-2, 3.21%; and HAstV-3, 1.60%. In China, the prevalence of the HAstV serotypes HAstV-1, HAstV-5, HAstV-3, and HastV-8 strains was 94%, 3%, 2%, and 1% of the HAstVs recovered (n=101), respectively. In Japan, the serotype distribution for HAstV-1, HAstV-4, HAstV-8, HastV-3, and HastV-5 strains was 84%, 8%, 4%, 3%, and 1% of the HAstVs recovered (n=141), respectively. In Thailand, 3 serotypes, i.e., HAstV-1, 2, and 5, were most prevalent and detected at similar frequencies (Fig. 2).
In Mexico, the prevalence of HAstV-1 was low (10%) compared to that of HAstV-2 (42%), HAstV-4 (23%), HAstV-3 (13%), HAstV-5 (6%), and HAstV-7 (6%)17), and a recent study in Madagascar reported a high prevalence of the unusual HAstV-8 strains38).
HAstV-1 strains could be classified into 4 lineages (1a to 1d), with the majority of Korean HAstV strains being clustered into lineage 1a (65.78%). In 2002, 91.67% of HAstV-1 strains were type 1a, but this prevalence significantly decreased during the following years, reaching 33% in 2007 (P<0.01) (Table 2). Studies conducted in Spain, Germany, Brazil, Vietnam, Japan, and China have indicated that HAstV-1d is the predominant type in these countries37).
HAstV is frequently associated with hospital-acquired gastroenteritis, including cases in immunocompromised patients who are known to excrete HAstVs for prolonged periods of time. Therefore, appropriate and effective isolation is essential in hospitals39). Control measures for outbreaks of viral gastroenteritis should focus on the removal of an ongoing common source of infection (e.g., an ill food handler or contamination of a water supply) and on the interruption of person-to-person transmission. HAstV infections usually resolve without specific treatment; some younger children or elderly patients, however, may require fluid replacement. In the future, immunization may play an important role for preventing HAstV infections.
HAstV is one of the most common causes of mild diarrhea, is very important as a nosocomial agent, and can cause outbreaks and hospitalizations. For adequate prevention strategies, including vaccine development, an improved understanding of the fundamentals of immunity to AstV such as the protective role of antibodies should be established. Other important areas for future research should focus on improving diagnostic assays for the detection of AstVs from human and environmental samples. In addition, detailed descriptions of clinical outcomes are required to assess the economic burden of AstV-related diseases in children.
Acknowledgment
This study was supported by an intramural fund from the National Institutes of Health (NIH-091-4851-300), Republic of Korea. The authors are grateful to the staff at 16 local public health institute laboratories and sentinel hospital participants in the surveillance program for acute gastroenteritis in Korea.
References
1. Glass RI, Noel J, Mitchell D, Herrmann JE, Blacklow NR, Pickering LK, et al. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch Virol Suppl 1996;12:287–300.


2. Matsui SM, Greenberg HB. Knipe DM, Howley PM,Astroviruses. editors. Fields virology. 2001;4th ed. Philadelphia: Lippincott Williams & Wilkins, :875–894.
3. Dalton RM, Roman ER, Negredo AA, Wilhelmi ID, Glass RI, Sánchez-Fauquier A. Astrovirus acute gastroenteritis among children in Madrid, Spain. Pediatr Infect Dis J 2002;21:1038–1041.


4. Gray JJ, Wreghitt TG, Cubitt WD, Elliot PR. An outbreak of gastroenteritis in a home for the elderly associated with astrovirus type 1 and human calicivirus. J Med Virol 1987;23:377–381.


5. Méndez-Toss M, Griffin DD, Calva J, Contreras JF, Puerto FI, Mota F, et al. Prevalence and genetic diversity of human astroviruses in Mexican children with symptomatic and asymptomatic infections. J Clin Microbiol 2004;42:151–157.



6. Coppo P, Scieux C, Ferchal F, Clauvel J, Lassoued K. Astrovirus enteritis in a chronic lymphocytic leukemia patient treated with fludarabine monophosphate. Ann Hematol 2000;79:43–45.


7. Cox GJ, Matsui SM, Lo RS, Hinds M, Bowden RA, Hackman RC, et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology 1994;107:1398–1407.


8. Belliot G, Laveran H, Monroe SS. Outbreak of gastroenteritis in military recruits associated with serotype 3 astrovirus infection. J Med Virol 1997;51:101–106.


9. Kurtz JB, Lee TW, Craig JW, Reed SE. Astrovirus infection in volunteers. J Med Virol 1979;3:221–230.


10. Oishi I, Yamazaki K, Kimoto T, Minekawa Y, Utagawa E, Yamazaki S, et al. A large outbreak of acute gastroenteritis associated with astrovirus among students and teachers in Osaka, Japan. J Infect Dis 1994;170:439–443.


11. Dennehy PH, Nelson SM, Spangenberger S, Noel JS, Monroe SS, Glass RI. A prospective case-control study of the role of astrovirus in acute diarrhea among hospitalized young children. J Infect Dis 2001;184:10–15.


12. Gaggero A, O'Ryan M, Noel JS, Glass RI, Monroe SS, Mamani N, et al. Prevalence of astrovirus infection among Chilean children with acute gastroenteritis. J Clin Microbiol 1998;36:3691–3693.



13. Palombo EA, Bishop RF. Annual incidence, serotype distribution, and genetic diversity of human astrovirus isolates from hospitalized children in Melbourne, Australia. J Clin Microbiol 1996;34:1750–1753.



14. Qiao H, Nilsson M, Abreu ER, Hedlund KO, Johansen K, Zaori G, et al. Viral diarrhea in children in Beijing, China. J Med Virol 1999;57:390–396.


15. Unicomb LE, Banu NN, Azim T, Islam A, Bardhan PK, Faruque AS, et al. Astrovirus infection in association with acute, persistent and nosocomial diarrhea in Bangladesh. Pediatr Infect Dis J 1998;17:611–614.


16. Pang XL, Vesikari T. Human astrovirus-associated gastroenteritis in children under 2 years of age followed prospectively during a rotavirus vaccine trial. Acta Paediatr 1999;88:532–536.


18. Mitchell DK, Matson DO, Jiang X, Berke T, Monroe SS, Carter MJ, et al. Molecular epidemiology of childhood astrovirus infection in child care centers. J Infect Dis 1999;180:514–517.


19. Marshall JA, Bruggink LD, Sturge K, Subasinghe N, Tan A, Hogg GG. Molecular features of astrovirus associated with a gastroenteritis outbreak in an aged-care centre. Eur J Clin Microbiol Infect Dis 2007;26:67–71.


20. Geigenmüller U, Chew T, Ginzton N, Matsui SM. Processing of nonstructural protein 1a of human astrovirus. J Virol 2002;76:2003–2008.



21. Kiang D, Matsui SM. Proteolytic processing of a human astrovirus nonstructural protein. J Gen Virol 2002;83(Pt 1): 25–34.


22. Méndez E, Fernández-Luna T, López S, Méndez-Toss M, Arias CF. Proteolytic processing of a serotype 8 human astrovirus ORF2 polyprotein. J Virol 2002;76:7996–8002.



23. Geigenmüller U, Ginzton NH, Matsui SM. Studies on intracellular processing of the capsid protein of human astrovirus serotype 1 in infected cells. J Gen Virol 2002;83(Pt 7): 1691–1695.


24. Lukashov VV, Goudsmit J. Evolutionary relationships among Astroviridae. J Gen Virol 2002;83(Pt 6): 1397–1405.


26. Bon F, Fascia P, Dauvergne M, Tenenbaum D, Planson H, Petion AM, et al. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J Clin Microbiol 1999;37:3055–3058.



27. Guerrero ML, Noel JS, Mitchell DK, Calva JJ, Morrow AL, Martínez J, et al. A prospective study of astrovirus diarrhea of infancy in Mexico City. Pediatr Infect Dis J 1998;17:723–727.


28. Morrow AL, Reves RR, West MS, Guerrero ML, Ruiz-Palacios GM, Pickering LK. Protection against infection with Giardia lamblia by breast-feeding in a cohort of Mexican infants. J Pediatr 1992;121:363–370.


29. Fang ZY, Sun YP, Ye XH, Wang H, Zhang Q, Duan ZJ, et al. Astrovirus infection among hospitalized children with acute diarrhea in seven regions of China, 1998-2005. Zhonghua Liu Xing Bing Xue Za Zhi 2006;27:673–676.

30. Jeong AY, Jeong HS, Jo MY, Jung SY, Lee MS, Lee JS, et al. Molecular epidemiology and genetic diversity of human astrovirus in South Korea from 2002 to 2007. Clin Microbiol Infect 2011;17:404–408.


31. Chan-it W, Thongprachum A, Okitsu S, Mizuguchi M, Ushijima H. Epidemiology and molecular characterization of sapovirus and astrovirus in Japan, 2008-2009. Jpn J Infect Dis 2010;63:302–303.


32. Pager CT, Steele AD. Astrovirus-associated diarrhea in South African adults. Clin Infect Dis 2002;35:1452–1453.


33. Shastri S, Doane AM, Gonzales J, Upadhyayula U, Bass DM. Prevalence of astroviruses in a children's hospital. J Clin Microbiol 1998;36:2571–2574.



34. Chikhi-Brachet R, Bon F, Toubiana L, Pothier P, Nicolas JC, Flahault A, et al. Virus diversity in a winter epidemic of acute diarrhea in France. J Clin Microbiol 2002;40:4266–4272.



35. Guix S, Caballero S, Villena C, Bartolomé R, Latorre C, Rabella N, et al. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J Clin Microbiol 2002;40:133–139.



36. Kang YH, Park YK, Ahn JB, Yeun JD, Jee YM. Identification of human astrovirus infections from stool samples with diarrhea in Korea. Arch Virol 2002;147:1821–1827.


37. Nguyen TA, Hoang L, Pham le D, Hoang KT, Mizuguchi M, Okitsu S, et al. Identification of human astrovirus infections among children with acute gastroenteritis in the Southern Part of Vietnam during 2005-2006. J Med Virol 2008;80:298–305.


Fig. 1
Phylogram including human astrovirus (HAstV) serotypes 1 to 8, feline AstV, porcine AstV, ovine AstV, turkey AstV, and avian nephritis virus. Neighbor-joining phylogenetic tree was constructed on the basis of nucleotide sequences of the capsid region of the HAstV genome. The numbers in the branches indicate the bootstrap values. Reference strains of HAstV selected from GenBank are indicated by accession numbers. The scale indicates nucleotide substitutions per position.
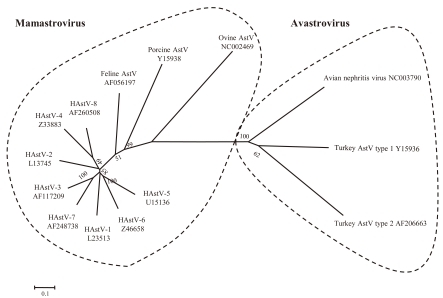
Fig. 2
Regional variation in the distribution of human astrovirus (HAstV) serotypes (n=436) ascertained by analysis of strains in Asia.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


