Article Contents
| Clin Exp Pediatr > Volume 68(6); 2025 |
|
Abstract
Adenomatous polyposis coli (APC) is a tumor suppressor gene expressed throughout the body. APC mutations increase the risk of malignancy and are often characterized by syndromes that encompass a spectrum of neoplastic manifestations, such as familial adenomatous polyposis (FAP). We present a rare case of palatal peripheral nerve sheath tumor in the context of APC gene mutation. A 17-year-old male with a significant history of FAP presented to our clinic with globus sensation for 5 months with increasing discomfort. Flexible nasolaryngoscopy revealed a pedunculated lesion attached to the posterior surface of the soft palate. Imaging was obtained and confirmed a soft tissue homogenous mass contiguous with the soft palate. Endoscopic-assisted transoral resection was performed and pathologic features were consistent with schwannoma. We also discuss the spectrum of benign neoplastic lesions. Current literature fails to describe pharyngeal masses in the setting of APC gene mutations. The purpose of this case report is to describe a patient presentation of a symptomatic pharyngeal tumor with a known APC gene mutation and explore the differential diagnoses that must be considered.
The adenomatous polyposis coli (APC) gene is a tumor suppressor gene on chromosome 5q21 that is expressed throughout the body and encodes a 312 kDa protein [1-3]. Its ability to act as a tumor suppressor relies on its interactions in the Wnt/beta-catenin signaling pathway. Functional APC proteins form a complex with beta-catenin resulting in phosphorylation, ubiquitination, and subsequent destruction of beta-catenin. In the presence of Wnt, beta-catenin is freed from the destruction complex and translocates to the nucleus to activate transcription factors [4,5]. Without a functional APC protein, beta-catenin is constitutively active resulting in abnormal gene transcription and neoplastic lesions.
APC-associated gene mutations may arise de novo or be inherited in an autosomal dominant pattern resulting in a variety of neoplastic growths [6]. Familial adenomatosis polyposis (FAP) is an autosomal dominant disorder notable for numerous APC-associated adenomatous polyps within the gastrointestinal tract. It is often described as a spectrum of syndromes that include Gardner and Turcot [7]. Gardner syndrome presents with FAP-associated intestinal polyps, osteomas, and desmoid tumors [8], while Turcot syndrome presents with intestinal polyps and central nervous system tumors [9]. Additional FAP-associated growths have been identified in the liver, abdominal wall, and odontomaxillary spaces [10,11].
APC-related manifestations presenting in the nasopharynx and neck are not well described, likely due to their rarity. The differential includes benign and malignant peripheral nerve sheath tumors that are promoted through the activation of Wnt signaling [12]. Nasopharyngeal angiofibromas and thyroid carcinomas have been reported as extracolonic manifestations of FAP [10,13-15]. Therefore, pharyngeal lesions in the setting of an APC-related genetic syndrome warrant further exploration. In this report, we describe our experience with a patient who presented with a rare palatal lesion and we discuss the role of immunohistochemical staining in discerning the diagnosis.
A 17-year-old male known for an APC 2434del4 mutation, colonic polyposis, a mandible osteoma, and poststreptococcal reactive arthritis presented to the Ohio State University Wexner Medical Center Otolaryngology clinic due to a globus sensation with increasing discomfort. Flexible nasolaryngoscopy showed a nasopharyngeal mass. Lab results at the time of presentation included elevated C-reactive protein and normal complement and lactate dehydrogenase levels. Computed tomography (CT) of the neck on August 7, 2023 demonstrated a 2.3×2.1×1.3-cm midline mass in the posterior pharynx contiguous with the soft palate (Fig. 1). Lung CT additionally showed four lower lobe 2–3 mm nodules bilaterally (left lower lobe: 3 mm, 3 mm, and 2 mm and right lower lobe: 3 mm).
Due to symptomatic manifestations and concern for malignancy, the patient underwent elective surgical removal of the mass. Intranasal and oral approaches were utilized. The mass was tan-yellow in color, friable, and bled upon manipulation (Fig. 2). Following removal, the tumor was sent to pathology for further characterization.
Pathology identified the mass as a 2.7×1.8×1.6-cm benign peripheral nerve sheath tumor that predominately showed features of a schwannoma (Fig. 3). The lobulated and nodular soft tissue mass was tan-pink to yellow in color and included a thin, mucosa-like fibromembranous covering and focal hemorrhagic material. Histology demonstrated hypocellular (Antoni B) and hypercellular (Antoni A) areas of ovoid cells in a myxoid to hyalinized stroma with a focal nuclear palisading pattern. No clear mitotic figures were appreciated. Mild degenerative cytologic atypia and cystic degeneration was present. Regions of necrosis showed ulceration of overlying squamous mucosa. Immunohistochemistry stains were diffusely positive for S-100 and calretinin. Peripheral staining was positive for CD34, glucose transporter protein 1 (Glut-1), and epithelial membrane antigen (EMA). The tumor was negative for Desmin, smooth muscle actin, and nuclear beta-catenin.
Throughout the surgical case, pathologic assessment, and write-up, appropriate patient information was protected and completely de-identified.
A variety of benign and malignant differential diagnoses were considered which included neurofibroma, neuroma, malignant peripheral sheath tumor, granular cell tumor, neural sheath myxoma, and Juvenile nasopharyngeal angiofibroma (JIA) (Table 1) [16-37]. Our patient’s mass was positive for S-100 indicating a peripheral nerve sheath tumor originating from Schwann cells [38]. However, schwannomas, neurofibromas, neuromas, malignant peripheral nerve sheath tumors, granular cell tumors, and neural sheath myxomas all demonstrate positive S-100 staining due to their cellular origination. Additional staining patterns and microscopic features were necessary to further differentiate which type of peripheral nerve sheath tumor was present.
Notably, our patient’s mass was also positive for GLUT-1 and EMA indicating encapsulation, seen as a thin rim of tissue demarcating the mass [16,17]. Of the masses on our differential, schwannomas and neurofibromas are most likely to be encapsulated. Although neuromas, granular cell tumors, and nerve sheath myxomas stain positively for S-100, neither are typically encapsulated as seen in this patient.
Moreover, malignant peripheral sheath tumors stain positively for S-100 and CD34 and demonstrate areas of necrosis as in our patient and often present with pseudoencapsulation; however, patterns of nuclear palisading are extremely uncommon. JIA is a common diagnosis of nasopharyngeal polyps in a younger patient population without a history of neoplastic growths. Yet, immunohistochemistry staining patterns and clinical features in our patient did not support the diagnosis of JIA.
Taken together, neurofibromas and schwannomas share a similar positive and negative staining profile to the mass removed from our patient and were highest on the differential diagnoses. However, patient demographics, histologic findings of encapsulation, and Antoni A and B bodies favor the diagnosis of a schwannoma (Table 2) [23-26,39,40].
This 17-year-old patient had a familial APC 2434del4 mutation and a history of colonic polyposis and a mandible osteoma, known manifestations of APC-related gene mutations. The nasopharyngeal mass excised from our patient with Gardner syndrome demonstrated multiple characteristics of a schwannoma, a rapidly growing benign peripheral nerve tumor originating from Schwann cells. Macroscopic excision demonstrated a clearly defined, exophytic mass with mucosal ulceration.
We additionally sought to broadly describe the immunohistochemical and histological features of nasopharyngeal neural tumors. Schwannomas often masquerade as other nerve sheath tumors and must be differentiated through staining patterns and microscopic features as demonstrated by this patient [18]. Distinctive positive immunohistochemical staining for S-100, CD34, Vimentin, GLUT-1, and EMA are often seen. Additionally, histologic growth patterns of Antoni A, described as hypercellular, nuclear palisading bodies, and Antoni B patterns, characterized as hypocellular arrangements accompanied by myxoid stroma support a schwannoma diagnosis.
Schwannomas may arise from anywhere along the peripheral nervous system. Rare locations of schwannoma presentation include the lungs [41], colon [42], and nasopharynx. Such diagnoses rely on immunohistochemical staining, commonly S-100 staining, and histopathologic examination, as seen in our patient. Notably, incidental lung masses were found on CT imaging our patient during the nasopharyngeal mass workup. The possibility of additional schwannomas present in this patient cannot be ignored and further workup is recommended.
Patients with nasopharyngeal schwannomas often present with long-standing complaints of nasal obstruction, snoring, and a globus sensation [43]. Rare symptoms include coughing, dysphagia, and even hearing loss [44]. Preoperative biopsy is not routinely performed due to its vascular nature in the nasopharynx. Proper operative planning including CT and magnetic resonance imaging can provide anatomic localization. Gold standard therapy is total excision resulting in a low risk of locoregional recurrence [45].
Recent advancements in genetic testing of tumors have allowed for identification of the entire genetic profiles. For instance, NF1, NF2, and CDKN2C are some of the most frequently mutated genes in spinal schwannomas [46]. Few cases of APC mutations manifesting into a schwannoma are known, and presentation of a nasopharyngeal schwannoma in the setting of FAP specifically has not been previously reported. One case report has identified a loss of APC within a cranial schwannoma via intragenetic markers [47]. However, the exact mechanism of APC mutations leading to schwannomas remains unclear. APC has shown a role in regulating axonal development and myelination in the peripheral nervous system [48]. A disruption in this mechanism may lead to the unregulated schwannoma growth. Further research is required to better understand the genetic landscape of schwannomas.
Postoperative follow-up studies for pharyngeal masses in the background of an APC mutation has not been established. However, the overall prognosis is optimistic. We recommend prompt removal of symptomatic pharyngeal masses due to the high risk of neoplastic transformation and to prevent airway obstruction. After complete resection of benign tumors, radiation and chemotherapy may be omitted [49]. In addition, typical surveillance and prophylactic procedures for patients with APC mutations should be continued.
In conclusion, schwannoma should be considered in patients with an exophytic nasopharyngeal mass. This case report highlights the differential diagnosis of a nasopharyngeal polyp and describes the novel presentation of a pharyngeal tumor demonstrating schwannoma features in the setting of a familial APC gene mutation.
Footnotes
Fig. 1.
Computed tomography neck demonstrated a midline mass (arrow) contiguous with the soft palate in the pharynx.

Fig. 2.
The neoplasm was appreciated intraoperatively from the oral cavity (A) and nasopharynx (B) on flexible laryngoscopy. (C) The area was reassessed in the operating room after the lesion was removed from the nasopharynx.
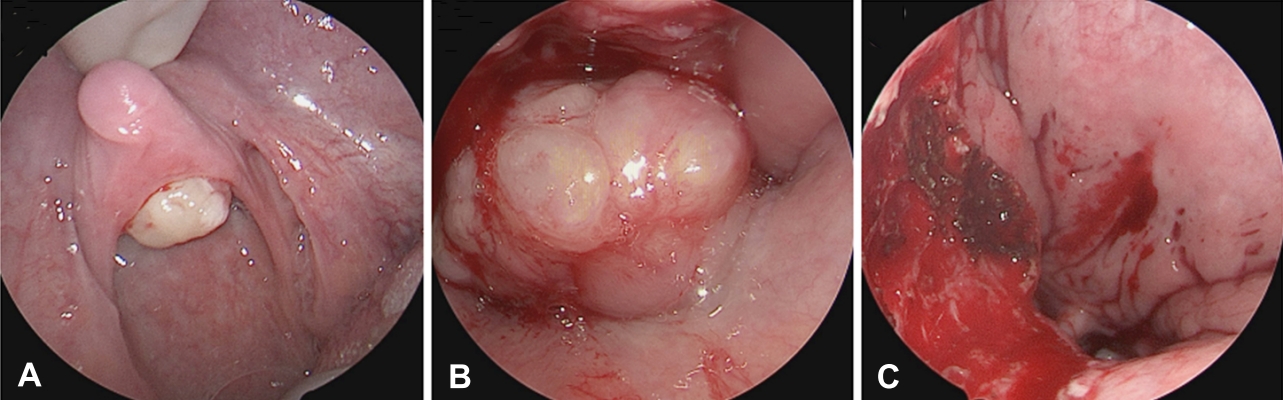
Fig. 3.
(A) Variable hypocellular and hypercellular areas with focally hyalinized stroma (H&E, ×10). (B) Verocay bodies with nuclear palisading (H&E, ×20). (C) S100 immunohistochemical stain with diffuse nuclear and cytoplasmic positivity (S100, ×20).
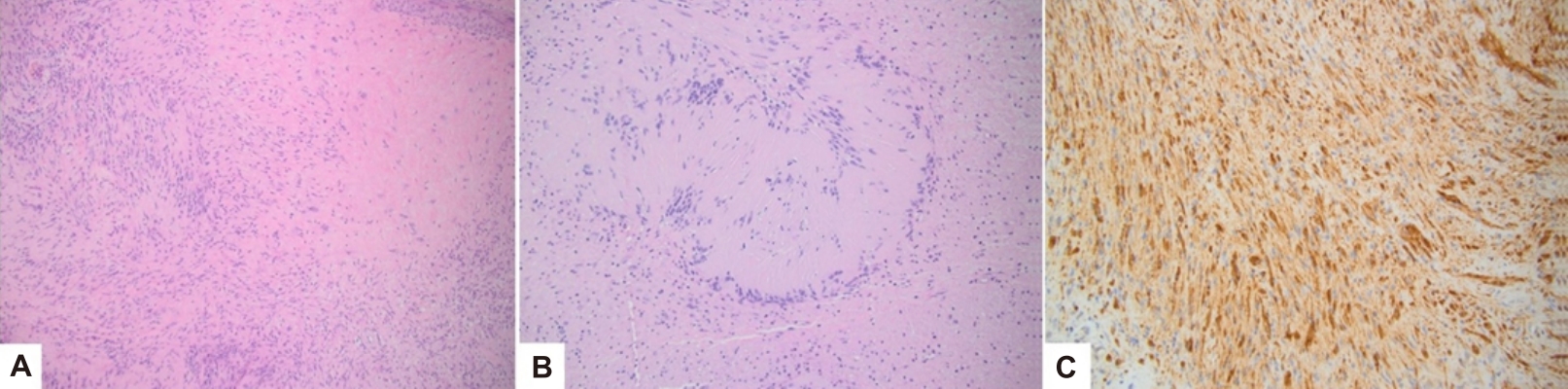
Table 1.
| Tumor | Positive stains | Negative stains | Features | Genetic associations | Average age range (yr) |
|---|---|---|---|---|---|
| Schwannoma | S-100 [18] | P53 [21] | Nuclear palisading [22] | NF-2 [24] | 15–50 [25] |
| CD34 [19] | Encapsulated [23] | ||||
| GFAP [17] | |||||
| Vimentin [17] | |||||
| EMA [17] | |||||
| GLUT-1 [16] | |||||
| SOX-10 [20] | |||||
| Neurofibroma | S-100 [19] | CD68 [26] | Soft [27] | NF-1 [26] | 20–60 [25] |
| CD34 [19] | Vimentin [26] | Less likely encapsulated [27] | |||
| SOX-10 [20] | SMA [26] | Plexiform variant [27] | |||
| Factor VIIIA | Desmin [26] | ||||
| Calretinin | |||||
| EMA [19] | |||||
| GLUT-1 [16] | |||||
| Podoplanin | |||||
| Low Ki67 [21] | |||||
| Neuroma | S-100 [28] | GFAP [29] | Traumatic [30] | 20–70 [25] | |
| GLUT-1 [16] | Nonulcerated [30] | ||||
| CD57 [23] | Nonencapsulated | ||||
| SOX-10 [20] | |||||
| Desmin | |||||
| Malignant peripheral sheath tumor | S-100 [19] | Calretinin [28] | Plexiform neurofibroma is a precursor lesion [31] | NF-1 [31] | |
| CD34 [19] | |||||
| CD56 [28] | Nuclear palisading is uncommon | ||||
| Vimentin [28] | Necrosis [32] | ||||
| SOX-10 [20] | Pseudoencapsulated [32] | ||||
| High Ki67 [21] | |||||
| P53 [21] | |||||
| Granular cell tumor | S-100 [33] | Nonencapsulated [33] | 25–50 [25] | ||
| P75 [33] | Tongue [33] | ||||
| Vimentin [33] | |||||
| Calretinin [33] | |||||
| NKI/C3 [33] | |||||
| Nerve sheath myxoma | S-100 [34] | EMA [34] | Firm [34] | 30–50 [34] | |
| NSE [34] | Non-encapsulated [34] | ||||
| SOX-10 | |||||
| Juvenile Nasopharyngeal Angiofibroma | VEGF [35] | Nasal obstruction [36] | 15–25 [37] | ||
| C-kit [35] | Male predominance [36] | ||||
| P53 [35] | Fibrocellular stroma [36] |
Table 2.
| Characteristic | Schwannoma | Neurofibroma |
|---|---|---|
| Epidemiology (age range) | 15–50 | 20–60 |
| Etiology | Sporadic | Sporadic |
| NF-2 [24] | NF-1 [26] | |
| Schwannomatosis [24] | ||
| Macroscopic | Encapsulated [23] | Soft |
| Lacks capsule [23] | ||
| Microscopic | Antoni A and Antoni B [39] | Spindle cells [23] |
| Hypercellular and hypocellular areas | Collagenous stroma [23] | |
| Myxoid areas [23] | ||
| Plexiform variant | Less common [24] | More common [25] |
| Malignant transformation | Rare [39] | Rare [25] |
| More likely in NF-1 [40] |
References
1. Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991;253:665–9.


2. Bhat RV, Baraban JM, Johnson RC, Eipper BA, Mains RE. High levels of expression of the tumor suppressor gene APC during development of the rat central nervous system. J Neurosci 1994;14:3059–71.



3. Mori T, Nagase H, Horii A, Miyoshi Y, Shimano T, Nakatsuru S, et al. Germ-line and somatic mutations of the APC gene in patients with Turcot syndrome and analysis of APC mutations in brain tumors. Genes Chromosomes Cancer 1994;9:168–72.


4. Schaefer KN, Peifer M. Wnt/beta-catenin signaling regulation and a role for biomolecular condensates. Dev Cell 2019;48:429–44.



5. Petersen GM, Slack J, Nakamura Y. Screening guidelines and premorbid diagnosis of familial adenomatous polyposis using linkage. Gastroenterology 1991;100:1658–64.


6. Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ. Hereditary colorectal polyposis and cancer syndromes: a primer on diagnosis and management. Am J Gastroenterol 2017;112:1509–25.


7. Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953;5:139–47.


8. Turcot J, Despres JP, St Pierre F. Malignant tumors of the central nervous system associated with familial polyposis of the colon: report of two cases. Dis Colon Rectum 1959;2:465–8.

9. Giardiello FM, Hamilton SR, Krush AJ, Offerhaus JA, Booker SV, Petersen GM. Nasopharyngeal angiofibroma in patients with familial adenomatous polyposis. Gastroenterology 1993;105:1550–2.


10. Wijn MA, Keller JJ, Giardiello FM, Brand HS. Oral and maxillofacial manifestations of familial adenomatous polyposis. Oral Dis 2007;13:360–5.


11. Truta B, Allen BA, Conrad PG, Kim YS, Berk T, Gallinger S, et al. Genotype and phenotype of patients with both familial adenomatous polyposis and thyroid carcinoma. Fam Cancer 2003;2:95–9.

12. Linos K, Sedivcová M, Cerna K, Sima R, Kazakov DV, Nazeer T, et al. Extra nuchal-type fibroma associated with elastosis, traumatic neuroma, a rare APC gene missense mutation, and a very rare MUTYH gene polymorphism: a case report and review of the literature*. J Cutan Pathol 2011;38:911–8.


13. Pegues J, McCown ET, Buck LS, Carron JD. Juvenile nasopharyngeal angiofibroma and familial adenomatous polyposis. Ear Nose Throat J 2021;100:1027S–1028S.



14. Gill J, Marin C, Amodeo D, Vidyarthi G, Boyd W. Granular cell tumor in a patient with familial adenomatous. Am J Gastroenterol 2010;105:S25–6.
15. Watson AL, Rahrmann EP, Moriarity BS, Choi K, Conboy CB, Greeley AD, et al. Canonical Wnt/β-catenin signaling drives human schwann cell transformation, progression, and tumor maintenance. Cancer Discov 2013;3674–89.


16. Salla JT, Johann AC, Lana AM, do Carmo MA, Nunes FD, Mesquita RA. Immunohistochemical study of GLUT-1 in oral peripheral nerve sheath tumors. Oral Dis 2008;14:510–3.


17. Deruaz JP, Janzer RC, Costa J. Cellular schwannomas of the intracranial and intraspinal compartment: morphological and immunological characteristics compared with classical benign schwannomas. J Neuropathol Exp Neurol 1993;52:114–8.


18. Alam K, Jain A, Misra A, Khan AH. Cellular schwannoma masquerading as malignant peripheral nerve sheath tumour: a diagnostic dilemma. BMJ Case Rep 2013;2013:bcr 2012008435.



19. Hirose T, Tani T, Shimada T, Ishizawa K, Shimada S, Sano T. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol 2003;16:293–8.


20. Miettinen M, McCue PA, Sarlomo-Rikala M, Biernat W, Czapiewski P, Kopczynski J, et al. Sox10-- a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol 2015;39:826–35.


21. Kindblom LG, Ahldén M, Meis-Kindblom JM, Stenman G. Immunohistochemical and molecular analysis of p53, MDM2, proliferating cell nuclear antigen and Ki67 in benign and malignant peripheral nerve sheath tumours. Virchows Arch 1995;427:19–26.



23. Chrysomali E, Papanicolaou SI, Dekker NP, Regezi JA. Benign neural tumors of the oral cavity: a comparative immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:381–90.

24. Berg JC, Scheithauer BW, Spinner RJ, Allen CM, Koutlas IG. Plexiform schwannoma: a clinicopathologic overview with emphasis on the head and neck region. Hum Pathol 2008;39:633–40.


25. Tamiolakis P, Chrysomali E, Sklavounou-Andrikopoulou A, Nikitakis NG. Oral neural tumors: Clinicopathologic analysis of 157 cases and review of the literature. J Clin Exp Dent 2019;11:e721–31.



26. Alotaiby FM, Fitzpatrick S, Upadhyaya J, Islam MN, Cohen D, Bhattacharyya I. Demographic, clinical and histopathological features of oral neural neoplasms: a retrospective study. Head Neck Pathol 2019;13:208–14.




27. do Nascimento GJ, de Albuquerque Pires Rocha D, Galvão HC, de Lisboa Lopes Costa A, de Souza LB. A 38-year review of oral schwannomas and neurofibromas in a Brazilian population: clinical, histopathological and immunohistochemical study. Clin Oral Investig 2011;15:329–35.



28. Guo A, Liu A, Wei L, Song X. Malignant peripheral nerve sheath tumors: differentiation patterns and immunohistochemical features - a mini-review and our new findings. J Cancer 2012;3:303–9.



29. Koutlas IG, Scheithauer BW. Palisaded encapsulated ("solitary circumscribed") neuroma of the oral cavity: a review of 55 cases. Head Neck Pathol 2010;4:15–26.




30. Jham BC, Costa NL, Batista AC, Mendonça EF. Traumatic neuroma of the mandible: a case report with spontaneous remission. J Clin Exp Dent 2014;6:e317–20.



31. Leroy K, Dumas V, Martin-Garcia N, Falzone MC, Voisin MC, Wechsler J, et al. Malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1: a clinicopathologic and molecular study of 17 patients. Arch Dermatol 2001;137:908–13.

32. Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol 2012;123:295–319.




33. Vered M, Carpenter WM, Buchner A. Granular cell tumor of the oral cavity: updated immunohistochemical profile. J Oral Pathol Med 2009;38:150–9.


34. Rozza-de-Menezes RE, Andrade RM, Israel MS, Gonçalves Cunha KS. Intraoral nerve sheath myxoma: case report and systematic review of the literature. Head Neck 2013;35:e397. –404.

35. Mishra A, Jaiswal R, Amita P, Mishra SC. Molecular interactions in juvenile nasopharyngeal angiofibroma: preliminary signature and relevant review. Eur Arch Otorhinolaryngol 2019;276:93–100.



36. Makhasana JA, Kulkarni MA, Vaze S, Shroff AS. Juvenile nasopharyngeal angiofibroma. J Oral Maxillofac Pathol 2016;20:330.



37. Coutinho-Camillo CM, Brentani MM, Nagai MA. Genetic alterations in juvenile nasopharyngeal angiofibromas. Head Neck 2008;30:390–400.


38. Mata M, Alessi D, Fink DJ. S100 is preferentially distributed in myelin-forming Schwann cells. J Neurocytol 1990;19:432–42.



39. Santos PP, Freitas VS, Pinto LP, Freitas Rde A, de Souza LB. Clinicopathologic analysis of 7 cases of oral schwannoma and review of the literature. Ann Diagn Pathol 2010;14:235–9.


40. Javed F, Ramalingam S, Ahmed HB, Gupta B, Sundar C, Qadri T, Al-Hezaimi K, et al. Oral manifestations in patients with neurofibromatosis type-1: a comprehensive literature review. Crit Rev Oncol Hematol 2014;91:123–9.


41. Walvir NM, Makhdoomi RH, Zargar M, Aiman A. Lung schwannomas, an unusual entity: a series of five cases. Lung India 2023;40:70–4.

42. Zainaldeen BA, Alaus AS, AlKooheji M, Alkhuzaie J. Schwannoma of the sigmoid colon: a rare case. Cureus 2024;16:e53140.



43. Somasekhar LS, Ramya S. Sinonasal schwannoma with secondary changes. Indian J Otolaryngol Head Neck Surg 2008;60:274–6.




44. Moukarbel RV, Sabri AN. Current management of head and neck schwannomas. Curr Opin Otolaryngol Head and Neck Surg 2005;13:117–22.

45. Batsakis JG, Sneige N. Parapharyngeal and retropharyngeal space diseases. Ann Otol Rhinol Laryngol 1989;98(4 Pt 1): 320–1.



46. Gao X, Zhang L, Jia Q, Tang L. Whole genome sequencing identifies key genes in spinal schwannoma. Front Genet 2020;11:507816.



47. Pećina-Slaus N. Loss of heterozygosity of the APC gene in a case of vestibular schwannoma assessed by two intragenetic markers. Acta Clin Croat 2012;51:643–7.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


