Article Contents
| Clin Exp Pediatr > Volume 65(12); 2022 |
|
Abstract
Purpose
Due to the prevalence of functional abdominal pain (FAP) and the importance of probiotics, this study aimed to compare the ability of 2 probiotics to reduce and improve FAP in children.
Methods
This open-label randomized clinical trial included 116 children aged 5–15 years with FAPP who met the ROME-4 criteria and were referred to the gastrointestinal clinic of Amir-Kabir Hospital in Arak in 2020–2021. The children were randomly allocated to receive polymicrobial probiotic (PMP group) or mono-strain probiotic (MSP group) once daily for 4 weeks. The standard Wong-Baker Faces scale was used to assess symptom severity.
Results
Of the 116 subjects, 62 (53.5%) were boys; the mean participant age was 7.39 years (standard deviation, 3.4 years). A significant intergroup difference (P=0.003) was observed in pain severity; 10.34% of children in the PMP group had no pain, while all patients in the MSP group reported low-degree pain. There was no intergroup difference in mean pain score (P=0.466), but it decreased over time in both groups (P= 0.001).
Chronic abdominal pain is a common problem in childhood and its prevalence in children in the United States and Europe is about 3%–19%. Almost 90% of these children have no organic cause [1]. This condition was first defined by Apley and Naish in 1957 as recurrent abdominal pain and also as at least 3 episodes of abdominal pain lasting less than 5 minutes, severe enough to affect their activities and lasting more than 3 months [2,3].
In 1999, the Rome II Pediatric Criteria introduced the term abdominal pain-predominant functional gastrointestinal disorders (AP-FGIDs); these include functional dyspepsia, irritable bowel syndrome (IBS), abdominal migraine, functional abdominal pain (FAP), and functional abdominal pain syndrome [4]. In order to meet these criteria, symptoms must occur every week, more than 3 months before diagnosis. With the introduction of the Rome III in 2006, this criterion was reduced to 2 months [5].
In children with AP-FGID, quality of life scores is significantly reduced compared to healthy peers, and AP-FGIDs are the second leading cause of school absenteeism. In 29.1% of patients with chronic abdominal pain, the pain remains common even for more than 5 years, despite medical care [6,7].
The pathogenesis of AP-FGID is still unknown. Intestinal changes, increased visceral sensitivity, abnormal brain disorders, psychosocial disorders, and immune activation have been suggested as possible explanations for symptoms [8]. In addition, studies in the United States and Europe have reported that psychological symptoms, economic status, gastrointestinal complaints of parents, and single-parent and immigrant families are associated with chronic abdominal pain in children [9,10].
Studies in patients with functional abdominal pain, or IBS, show intestinal microbiological changes by decreasing the number of lactobacilli and bifidobacteria in patients with IBS, and the predominant species, such as bacterioids and bifidobacteria, change to Clostridium. Probiotics have been defined as "living microorganisms" that, when used in sufficient quantities, affect host health, and have been proposed as a treatment for functional gastrointestinal disorders (FGD) [8].
While the exact mechanism of probiotics in patients with FGD is not known, several mechanisms have been proposed [11]. In addition, it has been shown that intestinal probiotics can directly affect bowel movements by affecting intestinal motility and modulation of intestinal pain, immune response, and nutrient processing. In adults, some clinical probiotic strains are more effective in treating IBS than placebo, but there are limited data in children [7).
Recent findings indicate that intestinal motility is significantly improved by supplementation with this probiotic [12,13]. Finding effective treatment for functional abdominal pain reduces heavy costs, reduces frequent visits to the doctor and improve the function of children with FAP. Also, chronic abdominal pain in children causes considerable anxiety and worry in the family and parents. The large intestine contains harmful bacteria, but the amount of beneficial bacteria must make up at least 85% of the total intestinal microorganisms to ensure the health of the host. The intestinal microbial flora is highly dependent on the food used, so the intestinal microbial flora can be altered and beneficial microbes replaced by harmful ones [14]. Due to the prevalence of FAP and the importance of probiotics, the aim of our study was to compare the effect of 2 probiotics on reducing and improving the FAP in children.
This study is an open-label randomized clinical trial conducted on 116 children aged 5 to 15 years with functional abdominal pain who met the ROME-4 criteria, referred to the gastrointestinal clinic of Amir-Kabir Hospital in Arak in 2020–2021. The children were randomly allocated into 2 groups including PMP and MSP. At the beginning of the study, the necessary explanations about FAP and this study and its benefits and side effects were given and then, if they having inclusion criteria, entered the study. This study was approved by the Ethical Committee of Arak University of Medical Sciences (ethical code: IR.ARAKMU. REC.1399.131) on June 28, 2020 and a written informed consent was obtained from the parents. The study protocol was registered in the Iranian Registry of Clinical Trials (registration No. IRCT20200806048325N1) on September 29, 2020 and updated on February 13, 2021. The CONSORT Statement (CONsolidated Standards of Reporting Trials) was followed to design and report the trial. Patient’s enrollment was continued from September 2020 to March 2021.
The patients selected for the study had FAP, which was diagnosed by a pediatric gastroenterologist. Inclusion criteria were functional abdominal pain according to the Rome 4 criterion, having parents' consent to participate in the study and age 5 to 15 years. Exclusion criteria included: (1) organic pain warning signs (includes age under 5, right upper and right lower abdominal pain, failure to thrive), (2) having organic disease (urinary tract infection, celiac disease, parasites, etc.) and other chronic diseases, (3) significant abdominal pain that prevents the child eats, plays or stays in school or kindergarten.
In this study, patients were divided into 2 equal groups including PMP group and MSP sachet group. The first group was given PMP capsules containing 6×109 units of probiotics including Bifidobacterium lactis, Lactobacillus acidophilus,Bifidobacterium bifidum, and Lactobacillus rhamnosus once a day for 4 weeks. The other group was given a MSP sachet containing 8×108 active cells of lyophilised Lactobacillus reuteri daily for 4 weeks with water or baby food. During the intervention, drug use and possible side effects were assessed by telephone and, if necessary, patients were visited in person. Patients were then followed for 4 weeks after the intervention.
As mentioned earlier, patients were followed up for 4 weeks and the changes made in them were evaluated in terms of pain intensity and frequency of pain. These changes and other study variables were recorded in a checklist provided to parents. The standard Wong-Baker Faces scale [15,16] was used to assess the severity of symptoms. The Wong-Baker Faces scale was originally created by children to measure pain in children. Today, this scale is used worldwide to measure the severity of pain in people 3 years and older. This tool enables the researcher to better assess and manage pain in children.
Permuted balanced block randomization method was used to assign the participants into 2 groups either PMP or MSP sachet group. Random sequence was generated by online program (https://www.sealedenvelope.com/randomisation/simulation/). Considering that the number of groups was 2, the size of the blocks was 4. Because the pattern of selected blocks is different and the blocks are selected randomly, the researcher is not able to predict the random sequence, therefore; in this method the concealment is also guaranteed.
After entering the data in the software, to describe the quantitative and qualitative variables, mean (standard deviation) and number (percentage) were used, respectively as well as graph and tables, and for statistical analysis, likelihood ratio chi square test, 2 independent t test, and repeated measures analysis of variance (ANOVA) were used. The normal distribution of data was investigated using Shapiro-Wilk test and the results showed that the data follow the normal distribution. Mauchly test was used to check the sphericity assumption, but no deviation was observed and the data met this assumption. All tests were performed using Stata 13 (Stata Corp., College Station, TX, USA) and the significance level was considered less than 5%.
Patients’ recruitment, loss to follow up and exclusions after randomization are shown in Fig. 1. To select 116 patients to participate in this trial, 183 patients were screened for inclusion criteria, 45 of whom did not meet the inclusion criteria, and 22 declined to participate into the study (67 patients were excluded). Finally, 116 subjects were included in the study who were randomly divided into 2 study groups and were followed up until the end of the study and 58 subjects in each group were included in the analysis.
Out of 116 people, 62 (53.5%) were boys and 54 were girls (46.5%), which in PMP group, 30 (51.7%) and in MSP group, 32 (55.2%) were boys. The mean age of all participants was 7.39 years (standard deviation [SD], 3.4) and the mean age of participants in PMP and MSP group was 7.35 years (SD, 3.14) and 7.43 years (SD, 3.67), respectively. The mean pain score in the first week before the intervention in PMP and MSP group is estimated as 3.03 (SD, 0.58) and 2.79 (SD, 0.81), respectively.
As shown in Table 1, the 2 groups were compared in terms of pain changes, but no statistically significant difference was observed between the 2 groups (P=0.710). Pain severity after the intervention was also compared between the 2 groups and based on statistical tests, a significant difference (P=0.003) was observed between the 2 groups so that in the PMP group 10.34 % of children had no pain but in the MSP group, all patients reported low pain. There was no significant difference between the 2 groups in terms of pain position (P=0.120) and having stressors (P=0.096).
To measure the pain score, Wong-Baker Faces scale was used and the results presented in Table 2. According to Table 2, the mean pain score at different studied times did not show a significant difference between the 2 groups. Based on repeated measure ANOVA, there is no significant difference between the 2 groups in terms of mean pain score (P=0.466), but the mean pain score has been significantly decreasing over time in both groups (P=0.001). The frequency distribution of the diagnosis in the 2 groups is shown in Table 3, based on the results, the frequency of FAP between the 2 groups was higher than other cases.
Chronic abdominal pain is a common problem in childhood and its prevalence in children, therefore; this clinical trial aimed to compare the effect of 2 probiotics (PMP and MSP) on reducing and improving the FAP in children.
The main results of this study suggested that there is no difference between the PMP and MSP group in terms of mean pain score, but the mean pain score has been significantly decreasing over time in both groups. This suggests that both probiotics are equally effective in controlling pain in patients with FAP.
Gawrońska et al. [17] conducted a clinical trial to assess the efficacy of L. rhamnosus GG (LGG) for 4 weeks in compared to placebo group for treating functional abdominal pain disorders in school-aged children. They concluded that consuming LGG could increase the success rate in treating these patients, which was greater in children with IBS. This was consistent with our study, which showed the effectiveness of probiotics in patients with abdominal pain.
Francavilla et al. [11] examined the efficacy of Lactobacillus in children with functional abdominal pain and found that probiotics reduced the frequency of pain, which is also consistent with a present study.
Jadrešin et al. [1], in their study on children aged 4 to 18 years, examined the therapeutic effect of probiotics in treatment of FAP and IBS and observed that L. reuteri eventually reduced pain intensity and also it is significantly associated with more days without pain, which have also been shown in our trial that both probiotics, including PMP and MSP, can be effective in controlling pain in patients with FAP.
Romano et al. [18] studied the effect of L. reuteri in children aged 6–16 years with FAP and found that supplements containing probiotics reduced the severity of abdominal pain, and this effect was also seen in the follow-up period even without taking probiotics.
Wegh et al. [19] in a systematic review assessed the efficacy of probiotics in children aged 4 to 18 years with functional abdominal pain disorders (FAPD) or children aged 0 to 18 years with functional constipation and found that there was not enough evidences for the use of probiotics in FAPD and functional constipation, with the exception of L. rhamnosus GG, which reduces the frequency and severity of abdominal pain in children with IBS. The results of their study were different from the results obtained from our study.
Drouault-Holowacz et al. [20] in a double-blind trial surveyed the effects of probiotic combination containing 4 strains of lactic acid bacteria on symptoms in patients with IBS and they found that probiotic compounds did not show a significant difference in reducing IBS symptoms compared with the placebo group.
Probiotics combined with general management strategies may be helpful in the management of abdominal pain but the mechanisms of action are not clear. Alterations to commensal bacterial populations have been implicated in dysmotility and immunologic activation. Probiotics may improve gastrointestinal symptoms by restoring the microbial balance in the gut through metabolic competition with pathogens, by enhancing the intestine mucosal barrier, or by altering the intestinal inflammatory response [21].
The most effective probiotic strain, dose, and treatment duration is not known. Given that probiotics generally are safe, the decision to use a probiotic is typically based on the potential benefits, costs, and patients/family preferences. When the decision is made to try probiotics, we suggest commercial preparations of strains that have same evidence of benefit in gastrointestinal disease (e.g., L. rhamnosus, L. reuteri) [22].
One of the strengths of this study was that the effect of 2 different types of probiotics was studied simultaneously, while in most previous studies, the effect of one type of probiotic compared to placebo has been studied. Also, in this study we were able to follow up and examine all the participants. One of the major limitations of this study was that probiotics were given to patients' families and it was recommended that they be taken completely and regularly, while some patients may not have full adherence. The interested outcomes were also followed up by visit and phone, which following the patients by phone can have some degree of measurement error. Another limitation of this study was that we did not have a placebo control group and therefore it is recommended to design similar studies with a placebo group for better conclusions.
Although the number of painless children in PMP group was significantly higher than the MSP group, based on the results observed in this study, no significant difference was observed between the 2 groups in terms of pain score and following the use of both PMP and MSP, the symptoms of patients has decreased. To better conclusion, designing a study with a placebo group is recommended.
Acknowledgments
We would like to acknowledge the patients and their families to their contribution and to the research deputy of Arak University of Medical Sciences for his scientific and financial supports throughout the study.
Fig. 1.
Patient recruitment flowchart. PMP, polymicrobial probiotic; MSP, mono-strain probiotic. CONSORT, CONsolidated Standards of Reporting Trials. CONSORT 2010 flow diagram.
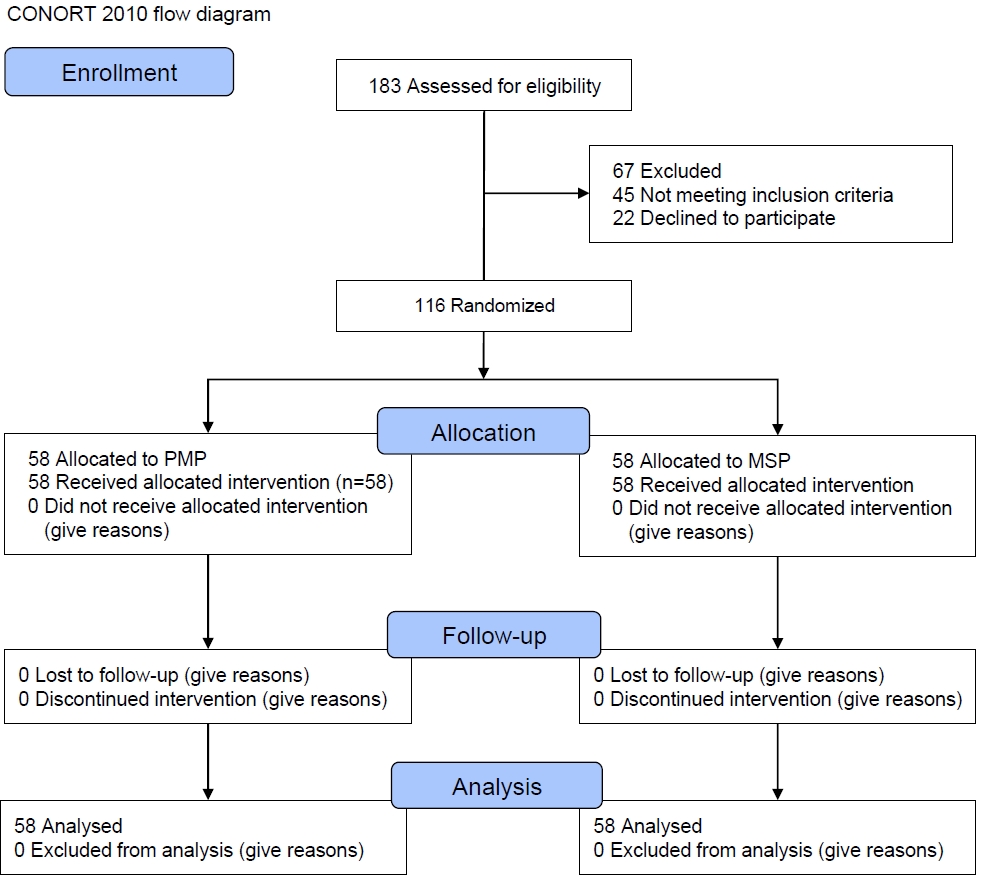
Table 1.
Patients’ clinical characteristics by study group
| Variable | PMP (N=58) | MSP (N=58) | P value |
|---|---|---|---|
| Age (yr) | 7.35±3.14 | 7.43±3.67 | 0.892a) |
| Sex | 0.710b) | ||
| Boy | 30 (51.72) | 32 (55.17) | |
| Girl | 28 (48.28) | 26 (44.83) | |
| Pain reduction | 0.710b) | ||
| No change | 29 (50.0) | 27 (46.5) | |
| Reduction of pain frequency | 29 (50.0) | 31 (53.4) | |
| Pain severity | 0.003b) | ||
| No pain | 6 (10.34) | 0 (0) | |
| Lower pain | 52 (89.66) | 58 (100) | |
| Pain position | 0.120b) | ||
| Periumbilical | 29 (50.0) | 17 (29.31) | |
| Hypogaster | 7 (12.07) | 8 (13.79) | |
| Epigaster | 4 (6.9) | 4 (6.9) | |
| Left lower quadrant | 18 (31.03) | 29 (50.0) | |
| Stressor | 0.096b) | ||
| Have | 13 (24.07) | 7 (12.07) | |
| Have not | 41 (75.93) | 51 (87.93) |
Table 2.
Mean pain score over time by study group
| Time | PMP (N=58) | MSP (N=58) | P valuea) |
|---|---|---|---|
| Baseline | 3.03±0.85 | 2.79±0.81 | 0.122 |
| First week | 2.56±0.91 | 2.56±0.90 | 0.999 |
| Third week | 2.22±0.95 | 2.17±0.81 | 0.755 |
| Fifth week | 1.93±0.89 | 1.91±0.84 | 0.915 |
| Seventh week | 1.34±0.68 | 1.24±0.65 | 0.409 |
References
1. Jadrešin O, Hojsak I, Mišak Z, Kekez AJ, Trbojević T, Ivković L, et al. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children: RCT study. J Pediatr Gastroenterol Nutr 2017;64:925–9.


3. Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, et al. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell Mol Gastroenterol Hepatol 2016;3:218–30.



4. Neuman MI, Fleisher GR, Drutz I, Wiley JF. Causes of acute abdominal pain in children and adolescents [Internet]. Waltham (MA): UpToDate; c2022 [cited 2022 Feb 12]. Available from: https://www.uptodate.com/contents/causes-of-acute-abdominal-pain-in-children-and-adolescents.
5. Jones MP, Faresjö Å, Beath A, Faresjö T, Ludvigsson J. Abdominal pain in children develops with age and increases with psychosocial factors. Clin Gastroenterol Hepatol 2020;18:360–7. e361.


6. Shulman RJ, Hollister EB, Cain K, Czyzewski DI, Self MM, Weidler EM, et al. Psyllium fiber reduces abdominal pain in children with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol 2017;15:712–9. e714.



7. Singh HK, Ee LC. Recurrent abdominal pain in children: is colonoscopy indicated? J Pediatr Gastroenterol Nutr 2019;68:214–7.


8. Prada-Arias M, Vázquez JL, Salgado-Barreira Á, Gómez-Veiras J, MonteroSánchez M, Fernández-Lorenzo JR. Diagnostic accuracy of fibrinogen to differentiate appendicitis from nonspecific abdominal pain in children. Am J Emerg Med 2017;35:66–70.


9. Boradyn KM, Przybyłowicz KE. Low FODMAP diet: a potential treatment of functional abdominal pain in children. Perspect Public Health 2017;137:314–5.



10. Trivić I, Niseteo T, Jadrešin O, Hojsak I. Use of probioticsin the treatment of functional abdominal pain in children-systematic review and metaanalysis. Eur J Pediatr 2021;180:339–51.



11. Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010;126:e1445–52.



12. He Y, Zhu L, Chen J, Tang X, Pan M, Yuan W, et al. Efficacy of probiotic compounds in relieving constipation and their colonization in gut microbiota. Molecules 2022;27:666.



13. Choi CH, Chang SK. Alteration of gut microbiota and efficacy of probiotics in functional constipation. J Neurogastroenterol Motil 2015;21:4–7.



14. Gonzalez V. Does the use of probiotics treat abdominal pain in children between the ages of 4 and 18 with irritable bowel syndrome? [Internet]. Philadelphia (PA): Philadelphia College of Osteopathic Medicine; 2017 [cited 2022 Feb 12]. Available from: https://digitalcommons.pcom.edu/cgi/viewcontent.cgi?article=1321&context=pa_systematic_reviews.
15. Garra G, Singer AJ, Domingo A, Thode HC Jr. The Wong-Baker Pain FACES Scale measures pain, not fear. Pediatr Emerg Care 2013;29:17–20.


16. Garra G, Singer AJ, Taira BR, Chohan J, Cardoz H, Chisena E, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med 2010;17:50–4.


17. Gawrońska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther 2007;25:177–84.


18. Romano C, Ferrau V, Cavataio F, Iacono G, Spina M, Lionetti E, et al. Lactobacillus reuteri in children with functional abdominal pain (FAP). J Paediatr Child Health 2014;50:E68–71.


19. Wegh CAM, Benninga MA, Tabbers MM. Effectiveness of probiotics in children with functional abdominal pain disorders and functional constipation: a systematic review. J Clin Gastroenterol 2018;52 Suppl 1. In: Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10 to 12, 2017:S10-26.
20. Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol 2008;32:147–52.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


