Introduction
Takayasu arteritis (TA) is a rare chronic inflammatory disease with a granulomatous panarteritis consisting of thickening, inflammatory infiltration, and hyperplasia of the arterial wall1). Clinical symptoms are various, ranging from nonspecific features to severe neurologic manifestations1). TA frequently presents with hypertension usually indicative of renal artery stenosis1).
Posterior reversible encephalopathy syndrome (PRES) is a rare neuroradiologic condition associated with headache, seizure, visual disturbances, and focal neurological deficit2). Most cases of PRES have been described in conditions with hypertension, immunosuppressive drug use, and autoimmune diseases2). Complete recovery is usually possible if the syndrome is appropriately diagnosed and the associated condition is well controlled2). The pathophysiology of PRES involves endothelial dysfunction, altered cerebral vasoregulation, and vasospasm with subsequent ischemia2). TA is a vasculitis with hypertension and hence can lead to the development of PRES. However, this association has rarely been described. We reported a case of a 5-year-old girl who presented with PRES caused by presumed TA.
Case report
A 5-year-old female visited the Emergency Department because of generalized tonic seizure. The duration of seizure was approximately 10 minutes and was controlled with anticonvulsant injections in another hospital. She was previously healthy and had no history of epilepsy, hypertension, or renal disease. The patient's mother was Korean and father was Japanese. She had no family history of hypertension, renal disease, or autoimmune disease. She suffered from a sudden onset of vomiting, headache and abdominal pain 1 week ago. She had no chest or back pain, and no history of limb claudication.
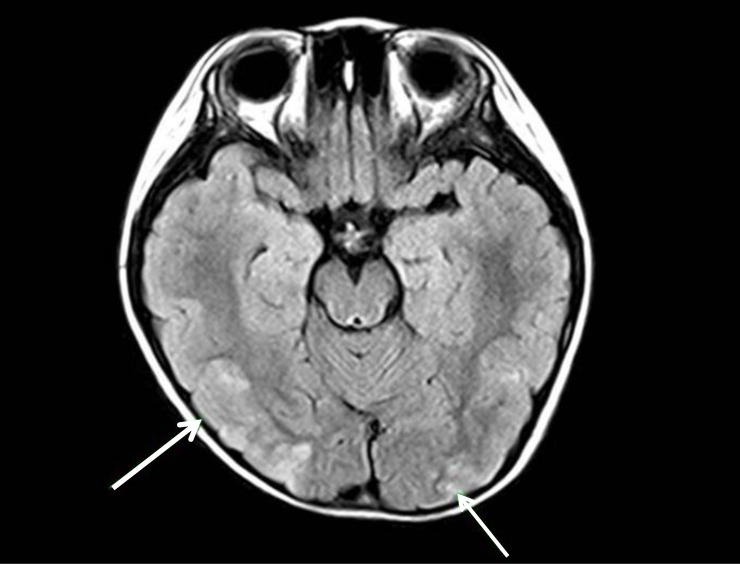
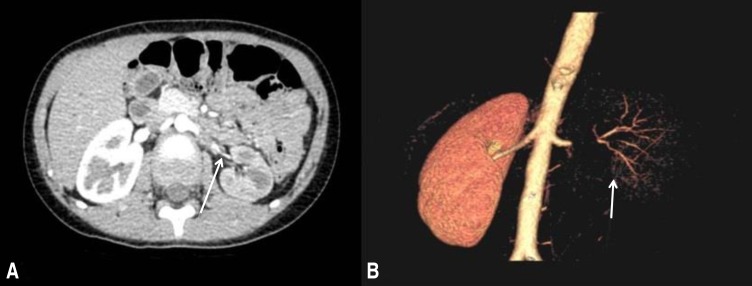
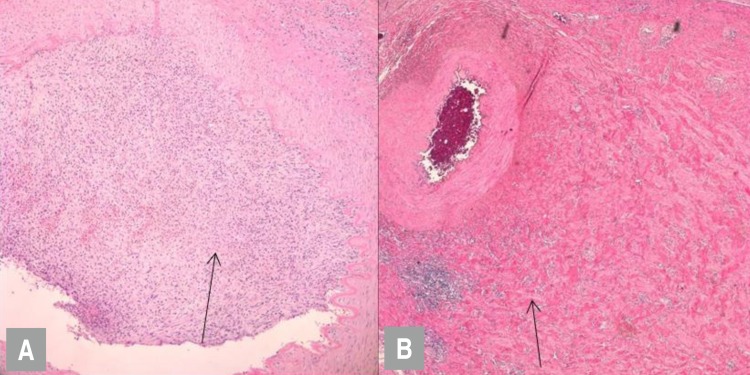
On admission, the subject's blood pressure was 170/103 mmHg, pulse rate was 94 beats per minute, and respiratory rate was 24 breaths per minute. She was afebrile. Height and body weight was 119.2 cm (95th percentile) and 22.3 kg (95th percentile), respectively. Auscultation revealed no gallop rhythm, abnormal heart sounds, or bruits over the abdomen. Peripheral pulses were full. There was no skin abnormality and the neurologic examination, including eyes was normal. Laboratory findings were as follows: hemoglobin, 14.8 g/dL; erythrocyte sedimentation rate (ESR), 17 mm/hr; C-reactive protein (CRP), 0.27 mg/dL; blood urea nitrogen/creatinine, 9.0/0.73 mg/dL; total protein/albumin, 8.4/5.2 g/dL; serum cholesterol, 188 mg/dL; IgG/IgA/IgM 929/123/115 mg/dL; serum sodium, 138 mEq/L; potassium, 4.0 mEq/L; and chloride, 95 mEq/L. Serum C3 and C4 levels were normal. Serum autoantibodies including antinuclear antibody, anti-dsDNA, antineutrophilic cytoplasmic antibody, and lupus anticoagulant were negative. The levels of serum aldosterone and renin activity were 85.1 ng/dL (normal range, 1ŌĆō27.3 ng/dL) and 59.9 ng/mL/hr (normal range, 0.15ŌĆō3.95 ng/mL/hr), respectively. Urinalysis showed a proteinuria (albumin 1+) without hematuria. Spot urine protein to creatinine ratio was 3.95 mg/mg creatinine. The findings of brain magnetic resonance imaging (MRI) were compatible with PRES (Fig. 1). The findings of electroencephalography were suggestive of diffuse cerebral dysfunction. Sustained hypertension with elevated plasma renin activity and normal renal function in the patient, suggested the possibility of renovascular hypertension. Computed tomography (CT) was performed to find out the cause of renovascular hypertension. CT angiography revealed a mass lesion encasing the left renal artery (Fig. 2). Dimercaptosuccinic acid (DMSA) renal scan showed no isotope uptake in the left kidney. After the admission, blood pressure was not controlled, and intravenous antihypertensive medication was injected and the dosage was gradually increased to control hypertension (nicardipine 1 mcg/kg/min and labetalol 3 mg/kg/hr). The patient showed the normal blood pressure (100/54 mmHg), the alert mentality and no seizure with intravenous antihypertensive medication. The pediatric nephrology and urology team made a collaborative decision to do perform a nephrectomy for evaluation of the mass lesion pathology and to control the hypertension. Thirteen days after her admission, left nephrectomy with laparoendoscopic single site surgery was performed. At 1-week follow-up after operation, the levels of serum aldosterone and renin activity were 1.9 ng/dL and 0.48 ng/mL, respectively. Serum creatinine was 0.54 mg/dL and proteinuria disappeared. The renal artery showed focal endovascular granulation tissue and perivascular fibromuscular proliferation on pathologic evaluation (Fig. 3). The kidney revealed diffuse ischemic change. These findings are not pathognomic, but suggestive of localized involvement of vasculitis, such as TA. Her blood pressure improved dramatically postoperatively, and she was discharged with angiotensin-converting-enzyme inhibitor (Enalapril 0.1 mg/kg/day) because she intermittently showed the blood pressure above 113/74 mmHg, which is the 95th percentile level of blood pressure by age and height percentile. Antihypertensive medication was discontinued after 1 month. Her blood pressure and renal function remained normal over 20 months without antihypertensive or immunosuppressant medication.
Discussion
TA is a systemic vasculitis of large vessels that mainly involves the aorta and its branches, usually presenting in the third decade of life3). Although the common presenting symptom of TA is hypertension, rare cases have reported hypertensive encephalopathy and PRES as presenting symptoms. The pathophysiology of PRES is explained by 2 conditions; hypertension leading to increased capillary filtration, or endothelial dysfunction resulting in an altered blood-brain barrier and the development of cerebral edema2). Our patient's inflammatory state and severe hypertension due to renal artery occlusion were presumably sufficient to develop PRES. Recent data from Camara-Lemarroy et al.4) showed that there are 10 previous cases of TA presenting with PRES in the literature, and the age of onset ranged from 9 to 29 years. Our case is the youngest age of the reported cases of TA presenting with PRES.
Diagnosis of TA during childhood remains challenging and may be delayed because of its rare incidence and the nonspecific symptoms3). An earlier case report by Mahmud et al.5) described a 5-year-old girl with TA who presented with a headache, generalized edema, hypertension, proteinuria, and minimally functioning kidneys. Aypar et al.6) reported about a 4-year-old girl with TA-induced hypertension treated with oral corticosteroids. Sandeep et al.7) described rare reports of TA in infants. Although these cases showed typical findings of TA according to angiography, our case revealed no evidence of other large vessel involvement beside the renal artery. The Takayasu Conference proposed a classification based on angiographic abnormalities, and similar cases of TA with only renal artery involvement are classified as TA type II8). Furthermore, there are some differences in vascular involvement and the incidence of aneurysms among different populations9). A comparative study has shown that Japanese patients more frequently have lesions at the aortic arch and/or its branches (99% of 96 cases), while the most common lesion in Korean (76% of 109 cases) and Indian patients (92% of 50 cases) is the abdominal aorta. Additionally, renal artery involvement is a potential manifestation of TA that has been found to occur in 60% of the patients in India and Korea9). The European League against Rheumatism/Paediatric Rheumatology European Society (EULAR/PReS) proposed that TA consists of an angiographic abnormality and at least one of the following four features: decreased pulses/claudication, blood pressure difference of >10 mmHg, bruit, and hypertension3). While the angiographic abnormality in this case was not a typical finding of TA, TA was a possible diagnosis according to the EULAR/PReS proposal, as the renal artery was affected and due to the pathologic findings in our case.
Because renovascular hypertension is usually be caused by fibromuscular dysplasia (FMD), it is important to differentiate TA from FMD. FMD is defined as a nonatherosclerotic, noninflammatory vascular disease and medial fibroplasia is a common finding3). On the other hand, TA is a chronic granulomatous inflammation and the vascular pathology in TA is characterized by segmental and patchy granulomatous inflammation of all three layers of the large vessels3). The findings of focal endovascular granulation tissue and perivascular fibromuscular proliferation in the renal artery were suggestive of a vasculitis-associated change, rather than FMD, in our case. The partial involvement of the circumference and definite inflammatory response seen in our case are unusual for FMD, hence the localized involvement of vasculitis, such as TA, was suspected. In our case, the lesion involved the renal artery. Vasculitis can be classified into large-vessel vasculitis including TA, polymyalgia rheumatic, and temporal arteritis. Among these large-vessel vasculitis classifications, only TA shows renal artery involvement-induced hypertension. Another possible diagnosis was a secondary change associated with septic emboli; however, our patient had no specific medical history suggestive of that diagnosis.
Recently, other diagnostic methods have been suggested for TA. MRI can be helpful because a thickened blood vessel wall is suggestive of the edema and inflammation seen in TA3). The other tools are positron emission tomography (PET) scans that are helpful in the diagnosis of inflammation in the blood vessel wall3,10). Therefore, further evaluation such as magnetic resonance angiography and PET may be necessary to confirm a TA diagnosis when a case shows the clinical manifestations associated with TA in the future.
Many patients with TA have an increased acute phase reactant such as ESR or CRP. Our case however, showed no increase of acute phase reactants. TA was two phases; an early systemic and a late occlusive phase1,3). The former is characterized by nonspecific symptoms such as fever, weight loss or fatigue, and increased ESR1,3). Half of the patients with TA are undiagnosed before the chronic phase in which symptoms caused by vascular occlusion, such as hypertension, predominante3). Total occlusion of the renal artery appeared to be chronic and the ESR was within the normal range, therefore our study subject was diagnosed in the chronic phase.
Renovascular hypertension caused by vasculitis is generally resistant to medical therapy and often requires additional invasive management, such as angioplasty or surgical bypass11). Recently, less invasive percutaneous transluminal angioplasty has become the first line therapy in such cases10). If it is unsuccessful or technically impossible, prompt renal artery bypass with the use of a saphenous vein graft with aortic inflow or relief of atypical coarctation with bypass grafting should be performed to lower blood pressure11). Nephrectomy was performed in our patient in order to control the blood pressure because the left kidney was nonfunctional according to the DMSA scan. Additionally, the mass lesion on CT angiography required pathologic evaluation. There have been reports that patients with TA showed a relapse when attempts are made to taper off the immunosuppressant or after successful angioplasty. Close monitoring for clinical findings associated with TA relapse will be necessary in our case.
This case suggested that childhood TA with renal artery involvement can present with PRES, despite the absence of typical TA symptoms. Appropriate diagnosis and immediate control of blood pressure are needed in order to have a good prognosis.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


