Article Contents
| Korean J Pediatr > Volume 59(6); 2016 |
|
Abstract
Purpose
Pulmonary arterial hypertension (PAH) leads to right ventricular failure (RVF) as well as an increase in pulmonary vascular resistance. Our purpose was to study the effect of sildenafil on right ventricular remodeling in a rat model of monocrotaline (MCT)-induced RVF.
Methods
The rats were distributed randomly into 3 groups. The control (C) group, the monocrotaline (M) group (MCT 60 mg/kg) and the sildenafil (S) group (MCT 60 mg/kg+ sildenafil 30 mg/kg/day for 28 days). Masson Trichrome staining was used for heart tissues. Western blot analysis and immunohistochemical staining were performed.
Results
The mean right ventricular pressure (RVP) was significantly lower in the S group at weeks 1, 2, and 4. The number of intra-acinar arteries and the medial wall thickness of the pulmonary arterioles significantly lessened in the S group at week 4. The collagen content also decreased in heart tissues in the S group at week 4. Protein expression levels of B-cell lymphoma-2 (Bcl-2)-associated X, caspase-3, Bcl-2, interleukin (IL)-6, matrix metalloproteinase (MMP)-2, endothelial nitric oxide synthase (eNOS), endothelin (ET)-1 and ET receptor A (ERA) in lung tissues greatly decreased in the S group at week 4 according to immunohistochemical staining. According to Western blotting, protein expression levels of troponin I, brain natriuretic peptide, caspase-3, Bcl-2, tumor necrosis factor-α, IL-6, MMP-2, eNOS, ET-1, and ERA in heart tissues greatly diminished in the S group at week 4.
Pulmonary arterial hypertension (PAH) affects all ages from newborns to adults. PAH leads to dysfunctions of both pulmonary vasculature and the heart. Chronic PAH results in significant peripheral and proximal arterial remodeling and right ventricular failure (RVF)1).
The pathophysiology in PAH is vasoconstriction from endothelial dysfunction, and an imbalance of vasoactive mediators. Remodeling of the pulmonary artery happens from an imbalance in the cell proliferation and apoptosis in the pulmonary vessel2).
Right ventricle (RV) adaptation and ventricular remodeling occurs after changes in pulmonary vasculature. Right ventricle hypertrophy (RVH) follows PAH because of compensatory mechanisms to the increased afterload. However, persistent overload results in RV dysfunction and failure3).
Ventricular pressure-volume relationships, changes in wall thickness and geometry are included in RV remodeling. Increased myocyte number, dimension and myocardial extracellular matrix can also be noted4). RV remodeling is connected with alteration in myocardial collagen content5). The larger amount of collagen is likely due to increased RV afterload as well as the paracrine factors to provoke RVH5). The levels of tumor necrosis factor (TNF)-α and collagen are increased in RVF6). Increased gene expressions of fetal contractile proteins and collagen were noted in the RV of rats7).
RV dilatation has been correlated with higher rates of apoptosis8). The primary crucial factor in the development of RVF is apoptosis.
Pharmacotherapy for PAH has been developing for the last 10 years to prevent the progress of the disease. Recently, there has been much interest in pulmonary vasodilators in therapies for PAH with heart failure. Inhaled nitric oxide (NO), endothelin antagonists and phosphodiesterase (PDE)-5 inhibitors have usually been used as a vasodilator in PAH patients9).
Sildenafil is a selective agent that relaxes pulmonary vascular smooth muscle by inhibiting cyclic guanosine monophosphate (cGMP) specific PDE. Decrease in pulmonary vascular resistance has been noted in sildenafil treatment in PAH10). Some research has shown that sildenafil improves endothelial function11) and reduces the pro-inflammatory cytokines such as TNF-α and interleukin (IL)-612) and inhibits the apoptosis progress in the PAH mechanism13).
However, the mechanisms of the effect of sildenafil on PAH patients associated with congestive heart failure (CHF) are not completely understood9,14). Sildenafil as a vasodilator may lead to decreased vascular pressure and tone in patients with CHF since vasoconstriction is contributed to an increase in ventricular filling pressure and pulmonary venous pressure15). Sildenafil decreases pulmonary arteriolar resistance and pressure in CHF by increasing NO availability because the defective release of NO may be a mechanism for the constriction of pulmonary vessels16).
There has been little research about the functional and structural changes of RV in PAH. The effects of current PAH therapies on the RV have not been thoroughly understood17).
The object of this research is to evaluate the effects of RV remodeling after sildenafil treatment in a rat model of monocrotaline (MCT)-induced RVF.
Six-week-old male Sprague-Dawley rats with a weight of 200–290 g, were used. All rats were housed in individual cages under standard conditions. RVF was revoked by subcutaneous injection of 60 mg/kg MCT (Sigma Chemicals, St. Louis, MO, USA) melted in 0.5 N HCl solution.
The rats were divided into three groups: C (control) group, M The rats were divided into three groups: C (control) group, M (MCT) group of subcutaneous injection of MCT (60 mg/kg), S (sildenafil, 30 mg/kg) group of daily gavage feeding of sildenafil for 28 days after MCT injection. We sacrificed 6 rats per each group at weeks 1, 2, and 4.
Protocol approval was received by the Institutional Animal Care and Use Committees of Ewha Womans University the School of Medicine of (approval number: 13-0226).
We weighed rats and monitored for general activity throughout the research period. The wet weights of RV, left ventricle (LV)+septum (S) and lung were gauged and RV to LV+S ratio [RV/(LV+S)] was calculated for an index of RVH.
We put the animals in the supine position with an arterial pressure line (Physiological Pressure Transducer, MLT1199; AD Instruments, Oxfordshire, UK). We inserted catheter in the external jugular vein to measure mean RVP. After estimating RVP, we measured pressure in the external carotid artery. Hemodynamic parameters were measured at weeks 1, 2, and 4.
Neutral buffered formalin (10% formalin in 0.08M sodium phosphate, pH 7.4) was used for lung tissue fixation before paraffin embedding. Three-µm-thick sections were made with Victoria blue staining. We captured more than 20 images of pulmonary arterioles per tissue section (diameter, 50–160 µm) at a magnification of ×400 using a microscopic digital camera and analysis program (analySIS, Olympus Soft Imaging Solutions, Singapore). We measured the medial wall thickness between the internal and external elastic lamina from two sides of muscular arteries (M1 and M2). The wall thickness was calculated as follows: % wall thickness=(M1+M2)/diameter×100. The number of intra acinar arteries was counted. A total of randomly selected microscopic fields per tissue section at a magnification (×200) were analyzed.
The heart tissues were stained with Masson's trichrome staining. This stain was used for distinguishing collagen from muscle tissues. Collagen contents were quantified using Image J developed by the National Institutes of Health.
We sliced formalin-fixed 4-µm section from paraffin embedded tissue blocks and then deparaffinizing with zylene and rehydrating by serial dilutions of alcohol (70%–100%) were done. Heat antigen retrieval was achieved at 100℃ for 10 minutes in microwave before incubation at 4℃ overnight.
Primary antibodies were used for B-cell lymphoma-2 (Bcl-2)-associated X (Bax), caspase-3, Bcl-2, TNF-α, IL-6, matrix metalloproteinase (MMP)-2, endothelial nitric oxide synthase (eNOS), endothelin receptor A (ERA) from Santacruz Biotechnology, Santa Cruz (Santa Cruz, CA, USA) and endothelin (ET)-1 from Abcam (Cambridge, UK). Slides were incubated with the biotinylated secondary antibodies for 30 minutes at 4℃ and then with a streptavidin (Dako, Kyoto, Japan). Color development was accomplished using 3-amino-9-ethylcarbazole or DAB as a chromogen. Densities were evaluated by using Image J and expressed in arbitrary units.
The tissue was homogenized and centrifuged. The supernatant was used for sodium dodecyl sulfate polyacrylamide gel electrophoresis. The proteins on the acrylamide gel were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) and the membranes were incubated at 4℃ overnight with the appropriated primary antibodies for troponin I, brain natriuretic peptide (BNP), caspase-3, Bcl-2, TNF- α, IL-6, MMP-2, eNOS, ERA from Santacruz Biotechnology and ET-1 from Abcam. Then, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. After the washing process the membrane was measured by a chemiluminescent reaction using an electrochemiluminescence-detection kit system from GE Healthcare (Amersham Bioscience, Piscataway, NJ, USA). The protein content was calculated with a Molecular Devices ELISA reader (Sunnyvale, CA, USA). Western blotting band intensity values were normalized by β-actin intensity.
Results were expressed as the mean±standard deviation. A Kruskal-Wallis test was used for the comparison of differences in the three groups and a Mann-Whitney test was used for between groups comparisons with Bonferroni correction. P value of <0.05 was considered statistically significant. SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. To determine whether immunohistochemical staining were statistically significant, we randomly selected five areas in each lung region of each group and measured mean staining densities.
The mean RVP was greatly higher in the M group in comparison with the C group at week 1 (n=6 per each group, 17.00±0.00 mmHg vs. 8.50±0.71 mmHg, P<0.05), at week 2 (n=6 per each group, 25.33±1.53 mmHg vs. 11.00±1.00 mmHg, P<0.05), and at week 4 (n=6 per each group, 39.67±8.50 mmHg vs. 10.00±0.00 mmHg, P<0.05). RVP was significantly reduced in the S group in comparison with the M group at week 1 (n=6 per each group, 13.00±1.00 mmHg vs. 17.00±0.00 mmHg, P<0.05), at week 2 (n=6 per each group, 13.00±1.00 mmHg vs. 25.33±1.53 mmHg, P<0.05) and at week 4 (n=6 per each group, 13.07±1.53 mmHg vs. 39.67±8.50 mmHg, P<0.05) (Table 1). There was no significant change in aorta pressure (Data is not shown).
The RV/BW ratio in the M group was greatly higher in comparison with the C group at week 2 (n=6 per each group, 0.81±0.08 g vs. 0.58±0.02 g, P<0.05) and at week 4 (n=6 per each group, 1.76±0.14 g vs. 0.62±0.06 g, P<0.05). The RV weight in the S group was greatly reduced in comparison with the M group at week 4 (n=6 per each group, 1.36±0.30 g vs. 1.76±0.14 g, P< 0.05). The RV/LV+S showed significant increase in M group in comparison with C group at week 2 (n=6 per each group, 0.41±0.06 g vs. 0.30±0.02 g, P<0.05) at week 4 (n=6 per each group, 0.75±0.06 g vs. 0.32±0.03 g, P<0.05) after MCT injection. Although the RV/LV+S in the S group was lowered in comparison with the M group, the result was not statistically significant (Table 2).
Fully muscularized arteries were seen in the pulmonary arterioles (>10µM, <100µM) in the M group and S group at weeks 2 and 4. Victoria blue staining identified inner and outer elastic layers of arteries (Fig. 1A).
The medial wall thickness (%) of pulmonary arterioles had increased significantly in the M group in comparison with the C group at week 2 (n=6 per each group, 44.99±4.84 vs. 25.71±7.85, P<0.05), at week 4 (n=6 per each group, 42.35±1.94 vs. 19.93±3.64, P<0.05). There was a significant decrease in the S group in comparison with the M group at week 2 (n=6 per each group, 35.41±3.13 vs. 28.44±4.61, P<0.05) and at week 4 (n=6 per each group, 33.75±0.80 vs. 42.35±1.94, P<0.05) (Fig. 1B).
The number of intra-acinar arteries significantly increased in the M group in comparison with the C group at week 1 (n=6 per each group, 1.48±0.16 vs. 1.44±0.29, P<0.05), at week 2 (1.86±0.24 vs. 1.23±0.08, P<0.05) and at week 4 (n=6 per each group, 2.01±0.30 vs. 1.24±0.11, P<0.05). There was a significant reduction of the number of intra acinar arteries in the S group in comparison with the M group at week 2 (n=6 per each group, 1.10±0.13 vs. 1.86±0.24, P<0.05) and at week 4 (n=6 per each group, 1.13±0.41 vs. 2.01±0.30, P<0.05) (Fig. 1C).
Since the Masson's Trichrome staining marks collagen-rich area in blue, the collagen-rich area was well visualized at week 2 and 4 (Fig. 2A) in each group. Collagen content (%) in the heart tissues significantly expanded in the M group in comparison with the C group at week 2 (n=6 per each group, 19%±2.55%, vs. 8.5%±1.41%, P<0.05) and at week 4 (n=6 per each group, 30%±3.54% vs. 11%±2.12%, P<0.05) after MCT injection and significantly reduced in the S group at week 4 (n=6 per each group, 25%±2.83 % vs. 30%±3.54%, P<0.05) (Fig. 2B).
The immunohistochemical staining assay by arbitrary unit revealed significantly enhanced expression of Bax, Caspase-3, Bcl-2, IL-6, MMP-2, eNOS, ET-1, ERA in the M group compared with the C group at week 4. In contrast, Bax, Caspase-3, Bcl-2, IL-6, MMP-2, eNOS, ET-1 and ERA significantly reduced in the S group at week 4 (Fig. 3A, B).
In western blot analyses of heart tissue, band intensity was normalized to the expression of the β-actin protein at each week. Protein expressions of troponin I (Fig. 4A), BNP (Fig. 4B), caspase-3 (Fig. 4C), Bcl-2 (Fig. 4D), TNF-α (Fig. 4E), IL-6 (Fig. 4F), MMP-2 (Fig. 4G), eNOS (Fig. 4H), ET-1 (Fig. 4I) and ERA (Fig. 4J) in the M group were significantly increased in comparison with the C group at week 4. Protein expressions of troponin I (Fig. 4A), BNP (Fig. 4B), Caspase-3 (Fig. 4C), Bcl-2 (Fig. 4D), TNF-α (Fig. 4E), IL-6 (Fig. 4F), MMP-2 (Fig. 4G), eNOS (Fig. 4H), ET-1 (Fig. 4I) and ERA (Fig. 4J) in heart tissues significantly lessened in the S group at week 4.
There was an unexpected increase in troponin I, BNP, TNF-α, and IL-6 protein expression levels in the C group. The small number of animals causes the unexpected increase in protein expression levels because of individual differences.
RV dysfunction (BNP), apoptosis (caspase-3, Bcl-2), inflammation (TNF-α, IL-6) and endothelial dysfunction (eNOS, ET-1, ERA) associated genes were significantly reduced after sildenafil treatment at week 4.
In this research, RVF and PAH were developed by intraperitoneal injection of MCT, which is selectively toxic to the endothelium of the pulmonary artery and causes PAH from 1 week after injection. We were able to confirm this by increased RVP and RV/LV+septum. RVF was confirmed by signs such as tachypnea, significant edema and ascites.
RVH is the underpinning of the functional and structural changes in RV remodeling. RV muscle mass is more susceptible to changes in afterload5). The functional and structural changes of cardiomyocytes happen because of stress on the myocardiocytes. Myocardiocytes under stress have a long contraction time and increased wall stress, following ventricular remodeling, contractile dysfunction and ventricular enlargement with pressure overload. Myocardiocyte changes include lengthened RV myocyte diameter, expanded mean myocardial cell volume, elongated myocyte length with shrunk cross-sectional areas and extended extracellular space5).
However, the critical mechanisms of change from hypertrophy to dilatation to cause RVF in PAH have not been well investigated3,18). Although incremented afterload is the first causal factor for RV adaptation in PAH17) neurohormonal signaling, oxidative stress, inflammation, ischemia, apoptosis and endothelial dysfunction may induce the development of RV dilatation and RVF17).
In our current research, increased inflammation (TNF-α and IL-6 expressions), apoptosis abnormality (caspase-3 and Bcl-2 expressions), endothelial dysfunction (eNOS, ET-1, and ERA expressions) and cardiac dysfunction (BNP and Troponin I expressions) were found in our MCT induced RVF model.
Collagen change has also been reported as an important pathophysiology in RV remodeling. Heart failure related to myocardiac infarction in a rat model showed an increased level of collagen gene expressions in the RV7). The increased levels of RV collagen and TNF-α were seen in failing hearts of patients with end-stage cardiomyopathy6).
In our study, collagen content in the heart tissues significantly increased at weeks 2 and 4 in MCT rats and significantly decreased after sildenafil treatment.
We investigated changes of RV protein expression levels after sildenafil administrations in MCT rats. The protein expressions related to endothelial cell dysfunction such as eNOS, ET-1, and ERA were significantly reduced after sildenafil treatment. ET and ERA have been noted to affect tissue remodeling. MMP-2 is an enzyme that degrades extracellular matrix. ET-1 plays a role as a vasoconstrictor and enhances cell proliferation and fibrogenesis by controlling MMP19). In our current research, the MMP-2 expression was augmented in the M group and lessened in the S group.
NO, produced by endothelial NO synthase, acts as a vasodilator in pulmonary vasculature. In some reports, the overproduction of eNOS in mice models prevents hypoxia-induced PAH20) and severe PAH was seen in eNOS deficient mice exposed to mild hypoxia21). On the other hand, there is a report that sildenafil elevates the cGMP in target cells and then decreases NOS activity and plasma NO level22). In our results, eNOS protein expressions were significantly increased in the M group compared with the C group and decreased after sildenafil treatment at week 4.
Inflammation and apoptosis are important mechanisms in the molecular change of the RVF in PAH. Increased inflammatory cytokines are known to increase pulmonary circulation and dilate RV of PAH patients3). In this study, the expressions of IL-6 and TNF-α increased in MCT induced RVF rats and decreased after sildenafil treatment. In addition, the failing RV may be subject to cardiomyocyte apoptosis13). There was also the result that the expressions of apoptosis related proteins, including caspase-3, bcl-2 augmented in the M group and reduced in the S group. Induction of apoptosis is another example of the cellular changes associated with RV remodeling. Bussani et al.8) compared apoptosis in myocardium with the degree of unfavorable cardiac remodeling. Apoptosis may be the primary critical factor to cause biventricular failure.
In our research, protein expressions of troponin I and BNP in the heart tissues significantly decreased at week 4 after sildenafil treatment. The troponin is one of the myocardial regulatory proteins and may be related to the pathobiology of heart failure23,24). Contractile dysfunction in heart failure is related to changes in regulatory and contractile and protein expression.
Ventricular BNP level is upregulated in cardiac failure and locally in the area around a myocardial infarct. BNP is valuable in the diagnosis and prognosis of heart failure. It is regarded to be the best biomarker in heart failure25). BNP is released by myocardial stretch from RV overload.
Hemodynamic effect of PDE-5 inhibitor on RVF with PAH has been recently found26). PDE-5 inhibitors may contribute to suppress RVH and improve RV function as well as decrease the RV afterload27). PDE-5 has been reported to be prominently upregulated in RVH myocardium4).
Sildenafil as a selective PDE-5 inhibitor is also known to prevent RV remodeling by a complex mechanism like modulating cGMP and calcium signaling28). Sildenafil increases cardiac index26) and prevents progressive chamber dilation, dysfunction and fibrosis of the heart with pre-existing hypertrophy and forces the contractility of cardiomyocytes28).
Sildenafil has also been reported to decrease pulmonary vascular resistance (PVR) and mean pulmonary artery pressure in patients with heart failure and PAH29). In our study, there was a significant decrease of RVP after sildenafil treatment. There were also improvements in RVH by estimating the decrease of RV weight in addition to improvement in pathology and gene expressions of the lung and heart.
Sildenafil may include the various and valuable combination of inotropic, antihypertrophic27) and afterload-decreasing effects on the RV without significantly influencing systemic hemodynamics30).
We considered 30 mg/kg of sildenafil was the optimal dose since the oral administration of sildenafil dose from 30 mg/kg to 100 mg/kg in rats seemed to be the effective regarding clearance. Meanwhile, we assessed that the dose of sildenafil below 30 mg/kg is less effective than the dose of sildenafil of 30 mg/kg considering the short half life according to previous data31).
Our gene expression studies of immunohistochemistry provided that the expressions of Bax, caspase-3, Bcl-2, IL-6, MMP-2, eNOS, ET-1, and ERA significantly decreased in the S group at week 4. In this study, we demonstrated that sildenafil improves endothelial dysfunction, apoptosis, inflammation and remodeling in the lung and heart tissues of MCT-induced rats.
However, further research is required to identify the exact mechanism of sildenafil effect. This study has some limitations. A longitudinal follow-up study is needed. Our study ended at week 4 because the survival rate in the M group was 56%, which is lower than that of the C group (100%) and the S group (100%) at week 4. There is also a lack of comprehensive assessment as to the diverse molecular changes involved in the effect of sildenafil treatment.
In conclusion, sildenafil treatment improved mean RVP, RVH and ventricular remodeling after sildenafil treatment. These results may have important implications for the experimental and therapeutic use of sildenafil.
Acknowledgment
This research received support from the Korean Medical Institute (2014) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A3004619).
Conflicts of interest
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Chesler NC, Roldan A, Vanderpool RR, Naeije R. How to measure pulmonary vascular and right ventricular function. Conf Proc IEEE Eng Med Biol Soc 2009;2009:177–180.



2. Wilkins MR. Pulmonary hypertension: the science behind the disease spectrum. Eur Respir Rev 2012;21:19–26.



3. Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013;62(25 Suppl): D22–D33.


4. Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007;116:238–248.


5. Kret M, Arora R. Pathophysiological basis of right ventricular remodeling. J Cardiovasc Pharmacol Ther 2007;12:5–14.


6. Kucuker SA, Stetson SJ, Becker KA, Akgul A, Loebe M, Lafuente JA, et al. Evidence of improved right ventricular structure after LVAD support in patients with end-stage cardiomyopathy. J Heart Lung Transplant 2004;23:28–35.


7. Yoshiyama M, Takeuchi K, Hanatani A, Shimada T, Takemoto Y, Shimizu N, et al. Effect of cilazapril on ventricular remodeling assessed by Doppler-echocardiographic assessment and cardiac gene expression. Cardiovasc Drugs Ther 1998;12:57–70.


8. Bussani R, Abbate A, Biondi-Zoccai GG, Dobrina A, Leone AM, Camilot D, et al. Right ventricular dilatation after left ventricular acute myocardial infarction is predictive of extremely high peri-infarctual apoptosis at postmortem examination in humans. J Clin Pathol 2003;56:672–676.



9. Lepore JJ, Maroo A, Bigatello LM, Dec GW, Zapol WM, Bloch KD, et al. Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: combined administration with inhaled nitric oxide. Chest 2005;127:1647–1653.


10. Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–2157.


11. Balarini CM, Leal MA, Gomes IB, Pereira TM, Gava AL, Meyrelles SS, et al. Sildenafil restores endothelial function in the apolipoprotein E knockout mouse. J Transl Med 2013;11:3



12. Toward TJ, Smith N, Broadley KJ. Effect of phosphodiesterase-5 inhibitor, sildenafil (Viagra), in animal models of airways disease. Am J Respir Crit Care Med 2004;169:227–234.


13. Schwartz BG, Levine LA, Comstock G, Stecher VJ, Kloner RA. Cardiac uses of phosphodiesterase-5 inhibitors. J Am Coll Cardiol 2012;59:9–15.


14. Urbanowicz T, Straburzyńska-Migaj E, Katyńska I, Araszkiewicz A, Oko-Sarnowska Z, Grajek S, et al. Sustained improvement of clinical status and pulmonary hypertension in patients with severe heart failure treated with sildenafil. Ann Transplant 2014;19:325–330.


15. Guazzi M, Tumminello G, Di Marco F, Guazzi MD. Influences of sildenafil on lung function and hemodynamics in patients with chronic heart failure. Clin Pharmacol Ther 2004;76:371–378.


16. Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 2000;102:1718–1723.


17. Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135:794–804.


18. McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 2005;115:1479–1491.



19. Abraham D, Ponticos M, Nagase H. Connective tissue remodeling: cross-talk between endothelins and matrix metalloproteinases. Curr Vasc Pharmacol 2005;3:369–379.


20. Ozaki M, Kawashima S, Yamashita T, Ohashi Y, Rikitake Y, Inoue N, et al. Reduced hypoxic pulmonary vascular remodeling by nitric oxide from the endothelium. Hypertension 2001;37:322–327.


21. Fagan KA, Fouty BW, Tyler RC, Morris KG Jr, Hepler LK, Sato K, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 1999;103:291–299.



22. Sirmagul B, Ilgin S, Atli O, Usanmaz SE, Demirel-Yilmaz E. Assessment of the endothelial functions in monocrotaline-induced pulmonary hypertension. Clin Exp Hypertens 2013;35:220–227.


23. Adamcova M, Sterba M, Simůnek T, Potacova A, Popelova O, Gersl V. Myocardial regulatory proteins and heart failure. Eur J Heart Fail 2006;8:333–342.


24. VanBuren P, Okada Y. Thin filament remodeling in failing myocardium. Heart Fail Rev 2005;10:199–209.


25. Panagopoulou V, Deftereos S, Kossyvakis C, Raisakis K, Giannopoulos G, Bouras G, et al. NTproBNP: an important biomarker in cardiac diseases. Curr Top Med Chem 2013;13:82–94.


26. Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002;105:2398–2403.


27. Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 2005;11:214–222.


28. Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, et al. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol 2009;53:207–215.



29. Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007;116:1555–1562.


30. Webster LJ, Michelakis ED, Davis T, Archer SL. Use of sildenafil for safe improvement of erectile function and quality of life in men with New York Heart Association classes II and III congestive heart failure: a prospective, placebo-controlled, double-blind crossover trial. Arch Intern Med 2004;164:514–520.


Fig. 1
Pulmonary arterioles stained with Victoria blue at the magnification of ×400 (A), medial wall thickness (B), and the number of intra-acinar arteries (C) in the 3 groups. The medial wall thickness of pulmonary arterioles significantly decreased in the S group in comparison with the M group at weeks 2 and 4 (panels A and B). There was a significant reduction in the number of intra-acinar arteries in the S group in comparison with the M group at weeks 2 and 4 (panel C). C, control; M, monocrotaline; S, sildenafil. *P<0.05: C vs. M at the corresponding time point. †P<0.05: M vs. S at the corresponding time point.
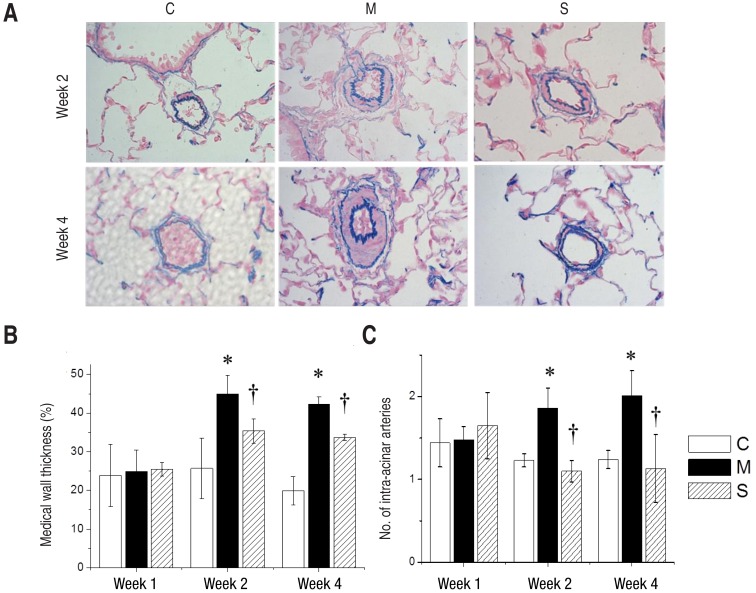
Fig. 2
Masson's trichrome staining of the heart tissues (×400) (A) and collagen content (B) in the 3 groups. The collagen-rich area of heart tissue was visualized well at weeks 2 and 4 (panel A) in each group. In comparison with group M, collagen content (%) of heart tissues significantly decreased in the S group after 4 weeks (panel B). C, control; M, monocrotaline; S, sildenafil. *P<0.05: C vs. M at the corresponding time point. †P<0.05: M vs. S at the corresponding time point.

Fig. 3
Immunohistochemical staining of lung tissues (A) and relative density (B) in 3 groups at week 4. The immunohistochemical staining assay by arbitrary unit revealed significantly reduced expressions of Bax, Caspase-3, Bcl-2, TNF-α, IL-6, MMP-2, eNOS, ET-1, and ERA in the S group compared with the M group at week 4 (×400). Bax, Bcl-2-associated X; Bcl, B-cell lymphoma; IL, interleukin; MMP, matrix metalloproteinase; eNOS, endothelial nitric oxide synthase; ET, endothelin; ERA, endothelin receptor A; C, control; M, monocrotaline; S, sildenafil. *P<0.05: C vs. M at the corresponding time point. †P<0.05: M vs. S at the corresponding time point.

Fig. 4
(A-K) Western blot analysis in the heart tissue at weeks 1, 2, and 4. Protein expressions of troponin I (A), BNP (B), caspase-3 (C), Bcl-2 (D), TNF-α (E), IL-6 (F), MMP-2 (G), eNOS (H), ET-1 (I), and ERA (J) in the heart tissues significantly lessened in the S group in comparison with the M group at week 4. BNP, brain natriuretic peptide; Bcl, B-cell lymphoma; TNF, tumor necrosis factor; IL, interleukin; MMP, matrix metalloproteinase; eNOS, endothelial nitric oxide synthase; ET, endothelin; ERA, endothelin receptor A; C, control; M, monocrotaline; S, sildenafil. *P<0.05: C vs. M at the corresponding time point. †P<0.05: M vs. S at the corresponding time point.
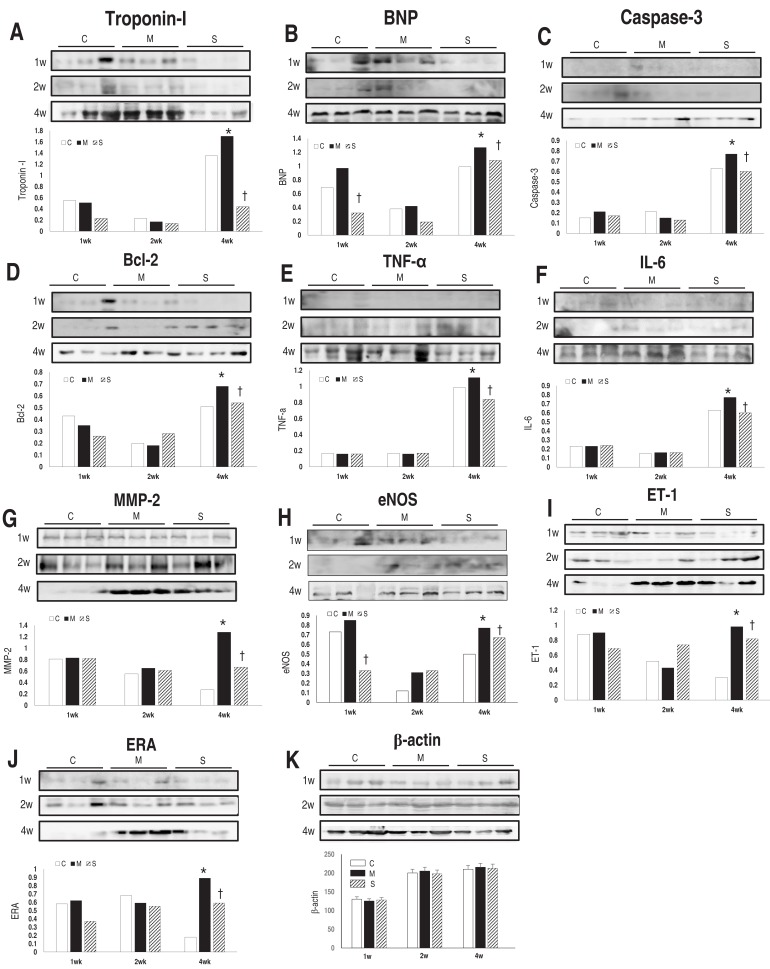
Table 1
The changes of right ventricular pressure at weeks 1, 2, and 4 in 3 groups
| Week | Control (C), (mmHg) | Monocrotaline (M), (mmHg) | Sildenafil (S), (mmHg) |
|---|---|---|---|
| 1 | 8.50±0.71 | 17.00±0.00* | 13.00±1.00 |
| 2 | 11.00±1.00 | 25.33±1.53* | 13.00±1.00 |
| 4 | 10.00±0.00 | 39.67±8.50* | 13.07±1.53 |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


