Article Contents
| Korean J Pediatr > Volume 59(6); 2016 |
|
Abstract
Purpose
Latent tuberculosis infection (LTBI) in young children may progress to severe active tuberculosis (TB) disease and serve as a reservoir for future transmission of TB disease. There are limited data on interferon-γ release assay (IGRA) performance in young children, which our research aims to address by investigating the usefulness of IGRA for the diagnosis of LTBI.
Methods
We performed a tuberculin skin test (TST) and IGRA on children who were younger than 18 years and were admitted to Chung-Ang University Hospital during May 2011–June 2015. Blood samples for IGRA were collected, processed, and interpreted according to manufacturer protocol.
Results
Among 149 children, 31 (20.8%) and 10 (6.7%) were diagnosed with LTBI and active pulmonary TB, respectively. In subjects lacking contact history with active TB patients, TST and IGRA results were positive in 41.4% (29 of 70) and 12.9% (9 of 70) subjects, respectively. The agreement (kappa) of TST and IGRA was 0.123. The control group, consisting of non-TB-infected subjects, showed no correlation between age and changes in interferon-γ concentration after nil antigen, TB-specific antigen, or mitogen stimulation in IGRAs (P=0.384, P=0.176, and P=0.077, respectively). In serial IGRAs, interferon-γ response to TB antigen increased in IGRA-positive LTBI subjects, but did not change considerably in initially IGRA-negative LTBI or control subjects.
Latent tuberculosis infection (LTBI) is defined as an infection with Mycobacterium tuberculosis without any manifestation of active tuberculosis (TB). This is not stationary, but active state in which immune system of host and M. tuberculosis are balanced. Therefore, when host immune function is compromised, LTBI may progress to active TB disease. Early diagnosis and treatment of LTBI are considered to be the most effective strategy for reducing the incidence of TB in the population1,2). Children are particularly vulnerable to development of severe disease and death following TB infection, and those with LTBI may serve as a reservoir for future transmission of TB disease3,4).
There is no way to directly detect the presence of latent M. tuberculosis infection. Instead, diagnosis of LTBI relies on measurement of host immune responses to M. tuberculosis antigens. Until the early 2000's, the only test available for LTBI was the tuberculin skin test (TST)2). Recently, two new diagnostic tests for LTBI have been approved, QuantiFERON–TB Gold (QFT) (Cellestis, a subsidiary of QIAGEN. Chadstone, Australia) and the T-SPOT.TB test (Oxford Immunotec Inc., Marlborough, MA, USA). Both tests are known as interferon-γ release assays (IGRAs) because they measure the release of interferon-γ from cells in vitro. Although it is unclear whether TST or IGRA is superior for detection of LTBI, the clinical importance of IGRA has been increasing5). Moreover, confirmation of LTBI in young children lacking an evident contact history with active TB patients, but with positive results for TST performed as routine practice, is problematic in the clinical setting.
In this study, we compared the diagnostic performance of IGRA and TST in LTBI. The influence of age on IGRA results was investigated, and the diagnostic usefulness of IGRA in non-TB contact children with positive TST result was explored.
Medical records of children younger than 18 years of age who were examined with IGRA between May 2011 and June 2015 were analyzed. Demographic and clinical data were collected using a specific form and then transferred into a database. Documentation of bacillus Calmette-Guérin (BCG) vaccination was confirmed using records from immunization diaries or an online site of Korean national immunization program (https://nip.cdc.go.kr). Parental consent to collect sensitive clinical data was obtained. The study protocol was approved by the Institutional Review Board of the Chung-Ang University Hospital (CAUH; IRB approval number C2013198-1158). Diagnosis of pulmonary TB was based on epidemiological, clinical, and radiological findings. TB infections were classified as latent according to the final diagnosis at the end of follow up and the following criteria: absence of clinical and radiological signs of active TB disease, positive TST results, and/or history of TB exposure.
TST was performed by trained nurses by intradermal injection of tuberculin-purified protein derivative RT23 SSI 2TU according to the Mantoux method. Trained physicians measured the diameter of induration 48–72 hours after administration. The test was considered to be positive if the diameter of induration was ≥10 mm, or if the diameter of induration was ≥15 mm in subjects lacking a TB contact history.
Peripheral blood samples for QuantiFERON–TB Gold in tube (QFT-IT) were collected, transferred to the laboratory, and processed within 3 hours according to the manufacturer's recommendations. IGRAs for children younger than 5 years old were performed with charge-free QFT-IT kit of research use, but processed equally to the routine test in CAUH. If the concentration of interferon-γ after stimulation with M. tuberculosis antigens was ≥0.35 IU/mL after subtraction of negative control value, the QFT-IT result was positive. The test result was negative if the result was <0.35 IU/mL with an adequate response to mitogen stimulation (positive control ≥0.50 IU/mL). The test result was considered indeterminate if the result was <0.35 IU/mL with a positive control <0.50 IU/mL. A test result <0.35 IU/ml with a negative control ≥8 IU/mL was considered invalid and excluded from the analysis.
Statistical analysis was performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Statistical significance was set at two-tailed P<0.05. To compare continuous variables of the study groups, Student t test was used. To evaluate discrete variables, Pearson chi-square and Fisher exact test were performed as appropriate. For the assessment of diagnostic agreement between tests, Cohen κ statistics were used.
A total of 149 children were enrolled in this study: 27 (18.1%) in 2011, 15 (10.1%) in 2012, 23 (15.4%) in 2013, 70 (47.0%) in 2014, and 14 (9.4%) in 2015. The median age of the subjects was 9.0 years (range, 0–18 years), and the number of subjects younger than 2 years old was 14 (9.4%), between 2–5 years old was 27 (18.1%), and 5 years old or older was 108 (72.5%). The ratio of males to females was 1.4:1. All but one subject had received BCG vaccination before 1 month of age. Subjects with contact history with active TB patient (TB-contact group) were 38 (25.5%), where index cases were primary relatives (n=26, 68.4%), secondary relatives (n=6, 15.8%), teachers (n=4, 10.5%), and classmates (n=2, 5.3%). Among the study subjects, 31 (20.8%) and 10 subjects (6.7%) were diagnosed with LTBI and active pulmonary TB, respectively.
Among subjects lacking contact history with an active TB patient (noncontact group; n=109, 73.2%), 39% (n=43) underwent TST for evaluation of continued respiratory symptoms, 18% (n=20) for evaluation of lymphadenopathy, 18% (n=20) for evaluation of nonspecific febrile illness, and 24% (n=26) as routine exam. Thirty-three of 41 subjects (80.5%) younger than 5 years were included in noncontact group. The TB contact history of 2 subjects was not available.
Data of induration size in TST were available in 108 subjects (72.5%): 36 subjects (94.7%) in TB-contact group and 70 subjects (64.2%) in noncontact group (P=0.000). In the TB-contact group, TST and IGRA results were both positive in 5 subjects (13.9%), and both test results were negative in 16 (41.7%). However, in 14 subjects (38.9%), TST results were positive and IGRA results were negative (Table 1). The percentage of positive TST and IGRA results was 52.8% (19 of 36) and 16.7% (6 of 36), respectively. The mean age of subjects with either positive or negative results of TST was 8.2±5.1 and 9.4±5.3 years, respectively (P=0.871). No significant difference was identified in age between positive and negative results of IGRA (12.0±4.0 years vs. 8.1±5.1 years, respectively, P=0.546). The agreement (kappa) of the 2 tests was 0.196.
In the noncontact group, only 8 subjects (11.4%) tested positive in both TST and IGRA. Most subjects (n=39, 55.7%) in this group tested negative in both tests. Nineteen subjects (27.1%) tested positive in TST and negative in IGRA, and 1 subject's (1.4%) results were the opposite (negative in TST and positive in IGRA). Three subjects (4.3%) (1 child aged 2 years and 2 children aged 1 year) had indeterminate results in IGRA: only 1 subject had positive TST results (induration, 14 mm). The positivity of TST and IGRA was 41.4% (29 of 70) and 12.9% (9 of 70), respectively. No significant differences were identified in age according to the results in TST and IGRA (P=0.665 and P=0.263, respectively). The agreement (kappa) of the 2 tests was 0.123.
TST results were available in 34 subjects (82.9%) in children aged younger than 5 years and 74 subjects (68.5%) in children aged 5 years or older (P=0.079). In the LTBI group (n=31) defined by the result of TST, only 9 (29.0%) had positive IGRA results. Among the subjects younger than 5 years (n=11, 35.5%), 8 subjects were non-TB contact children. All these subjects had negative (n=7) or indeterminate (n=1) results for IGRA. The mean age of subjects in the IGRA positive and negative group was 11.1±4.1 and 7.4±5.0 years, respectively (P=0.429). Induration size of TST was also not significantly different between the IGRA positive and negative group (11.7±6.5 mm vs. 13.4±5.2 mm, respectively, P=0.574).
In the control group (n=108), among those not diagnosed with LTBI or active TB, 32 subjects (29.6%) were younger than 5 years of age. Between the younger (<5 years of age) and older (≥5 years of age) age groups, values of interferon-γ released in response to negative control (nil: 0.22±0.25 IU/mL vs. 0.32±0.95 IU/mL, respectively), TB antigen (0.25±0.27 IU/mL vs. 0.43±1.24 IU/mL, respectively), and phytohemagglutinin (PHA: 10.91±6.36 IU/mL vs. 12.77±4.73 IU/mL, respectively) showed no significant differences (P=0.081, P=0.103, and P=0.438, respectively). In addition, the values of interferon-γ released in response to nil, TB antigen, and PHA were not correlated with age (R=0.085, P=0.384; R= 0.131, P=0.176; and R=0.171, P=0.077, respectively, Fig. 1).
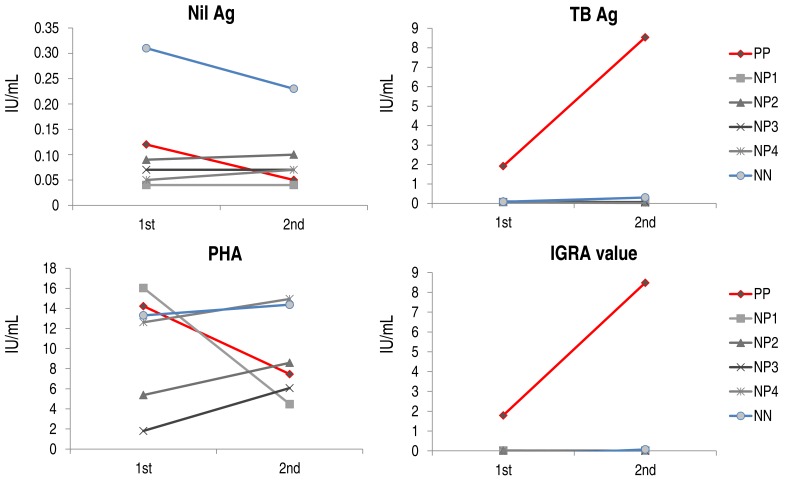
In six subjects, IGRA testing was repeated 34–63 days (median, 44.5 days) after initial testing, in which 1 subject had positive results in both IGRA and TST (PP), 1 subject had negative results in both tests (NN), and the remaining four subjects had negative IGRA results and positive TST results (NP1–4). Values for nil and PHA were decreased in the PP subject, but the value for TB antigen was significantly increased, resulting in the increased IGRA value (Fig. 2). In the subjects with negative IGRA results (NP1–4 and NN), nil values were not significantly changed and PHA values were slightly elevated in all but one subject, whose PHA value was decreased markedly. However, the TB antigen values and IGRA values were comparable between the 2 tests, regardless of TST results.
This study was performed to evaluate the performance of IGRA in diagnosing LTBI in children, particularly younger than 5 years of age, and non-TB contact children with positive TST results. We observed poor agreement between TST and IGRA results in young children. Interferon-γ response to various antigens was not decreased in children younger than 5 years of age and was not correlated with age. The incidence of indeterminate IGRA results was very low in this study.
The IGRA uses purified antigens from M. tuberculosis to stimulate peripheral blood lymphocytes to produce interferon-γ. A single specimen of peripheral blood is drawn and incubated overnight with specific antigens for M. tuberculosis. Interferon-γ production is then determined. A benefit of IGRAs is the avoidance of the booster phenomenon. TST results can be confounded by nontuberculous mycobacteria, history of BCG vaccination, competency of personnel administering the test, and reading of results. Despite these advantages of IGRA compared to the TST in the diagnosis of TB infection, the IGRA could not be actively used in children due to lack of accumulated clinical data. Previous studies conducted in Korean children mainly evaluated the value of TST and IGRA for TB contact screening in the limited situation with lacking a diagnostic gold standard6,7,8,9). In this study, we focused on the performance of IGRA in children younger than 5 years old and analyzed the individual response of three IGRA antigens separately.
Previous studies comparing IGRA and TST have demonstrated that IGRA is more specific than, and at least as sensitive as, TST for detecting LTBI in children as well as adults10,11). Other studies observed a strong correlation between positivity of IGRAs and a high TB exposure gradient, such as smear positivity, cavitary disease, and exposure time12,13). However, IGRA has been shown to have variable levels of agreement with TST in pediatric populations. The kappa value of the 2 tests was 0.063–0.693 in the previous Korean studies6,7,8,9), and was 0.196 in this study. Higher rates of discordance were reported in BCG-vaccinated than in non-BCG-vaccinated children in other studies14,15). Therefore, IGRAs are mainly recommended for increasing test specificity in children who have received a BCG vaccine, and for increasing sensitivity for diagnosing LTBI in patients at high risk of developing progression from LTBI to active TB disease, using with the TST16,17,18). Although the likelihood that prior BCG vaccination will interfere with the results of the TST has been shown to be minimal in a meta-analysis, only three of the 24 studies used in the analysis included children younger than 5 years of age, who were less than 1% of subjects in this study19). As well as the results of this meta-analysis cannot be applied to young children, false positivity of TST due to BCG vaccination might be higher in young children because the time interval between vaccination and testing was shorter in young children. Although guidance from the Centers for Disease Control and Prevention (CDC) indicates a preference for TST over IGRA in children younger than 5 years old, recent studies' findings support the preferential use of IGRAs over TSTs for diagnosing LTBI in BCG-vaccinated children two years of age and older20,21).
A main reason that IGRA is not recommended in children less than 5 years old is due to reports from previous studies suggesting an insufficient interferon-γ secretion response to the PHA being used as a positive control as well as to M. tuberculosis antigen in infants and children of this age group22,23,24). Therefore, there are concerns about the applicability of these assays to newborns and infants since decreased Interferon-γ production in response to various stimuli has been described in newborns25). This would potentially decrease the sensitivity of the IGRA, and increase the chance of false-negative assay results, or require re-evaluation of lower threshold values for positive responses. However, false negativity in young children has also been observed in TST as well26). TSTs are frequently misleading, because the results are usually negative during the first few weeks of M. tuberculosis infection. On the other hand, the usefulness of IGRAs in the evaluation of infants with suspected perinatal TB was reported27). The IGRA performed well in children less than 5 years of age, and results did not appear to be adversely affected in very young children17). Furthermore, there is no evidence that age adversely affects IGRA performance17,28). Also in the current study, the values of interferon-γ released in response to nil, TB antigen, and PHA showed no significant differences between the age groups, and were not correlated with age.
Additionally, clinicians have been concerned that using IGRA in young children might yield a high proportion of indeterminate results. The frequencies of indeterminate IGRA results in children vary greatly among studies (range, 0%–17%)15,16,17,21,23,28). In a previous study, age younger than 5 years was significantly associated with indeterminate results in IGRA23). Although indeterminate results were more common among the youngest children, recent studies conducted in younger children showed very low rates (0.5%–0.7%)17,21). In this study, the rate of indeterminate results was 2.0% (3 of 149). Indeterminate results in the early studies could be due to errors prior to analysis or clinical factors (e.g., low T-cell count, immunosuppression, or high background response due to recent viral infection). Moreover, most children with an indeterminate IGRA result were TST-negative and determined to be uninfected. Therefore, the risk of developing active TB among children with indeterminate results is very low15,17,21,28).
An additional problem in diagnosing latent TB infection is that screening of potential contact is currently limited to those within a family or a group of close contacts. The CDC discourages the use of diagnostic tests for LTBI among populations at a low risk for infection with M. tuberculosis because of the high rates of false positive results20). In Korea, the incidence of tuberculosis remains high, and population influx from areas of high TB prevalence such as China and Southeast Asia continues. In this situation, it is difficult to interpret positive TST test results in patients with no history of TB contact. The IGRA may be used as an adjunct to TST in such instances, but current data are insufficient. A key determinant of TB infection is the amount of time spent sharing room air with the index case. Infection of parents or caregivers is an exclusively important source of infection in young children. In this case, children with positive TST results without history of contact with an active TB patient in their family may indicate that there is a likelihood of false positives in this population.
In this study, IGRA results were rechecked in 6 subjects including 5 patients diagnosed with LTBI. Although we could not provide any conclusion due to very low number of subjects, interferon-γ level on TB antigen and IGRA value were both elevated only in the first IGRA positive subject. On the other hand, interferon-γ level of TB antigen and IGRA value were both not elevated in first IGRA-negative and TST-positive subjects (defined as patients with LTBI in this study) as well as in both test-negative subject (control; not LTBI). IGRA follow-up testing might be helpful in decreasing the number of false positive of TST results in BCG-vaccinated children, but further study with a large number of subjects will be needed.
A limiting factor in this study was that we could investigate a relatively small number of children who had not been in contact with TB-infected adults, but had positive TST results, particularly children younger than 5 years (n=8). The number of children who underwent repeated IGRA testing was also very small (n=6). In addition, the proportions of subjects with data of TST result were different between TB-contact group and noncontact group. Therefore, the selection bias might be introduced into the analyses. However, the analyses were performed separately in each groups, rather than the comparison between the 2 groups. Therefore, we expect that this bias would not significantly influence on the main outcomes in this study. Furthermore, this study showed results consistent with those of recently performed, well-designed clinical studies, and provided multidirectional comparative analyses. The results of this study would be confirmed by further research with larger study populations. In conclusion, this study found that interferon-γ releasing response was not influenced significantly by age, and that serial IGRA testing may improve the accuracy of LTBI diagnosis in children.
Acknowledgment
This study was supported by a 2013 research grant from the Korean Pediatric Society (Seokcheon Research Award).
Conflicts of interest
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Ellner JJ. Review: the immune response in human tuberculosis: implications for tuberculosis control. J Infect Dis 1997;176:1351–1359.


2. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015;372:2127–2135.


3. Kim JH. Treatment of latent tuberculous infection in children and adolescent. Korean J Pediatr 2009;52:519–528.

5. Lewinsohn DA, Lobato MN, Jereb JA. Interferon-gamma release assays: new diagnostic tests for Mycobacterium tuberculosis infection, and their use in children. Curr Opin Pediatr 2010;22:71–76.


6. Choi JW, Kim MS, Kim JH. Comparison of results between tuberculin skin test and QuantiFERON(R)-TB in-tube assay for diagnosis of latent tuberculosis infection in children and adolescents. Korean J Pediatr Infect Dis 2013;20:17–27.

7. Lee HW, Park HY, Ahn YM, Sohn KC. Clinical significance of interferon gamma release assay for diagnosis of tuberculosis in children. Korean J Pediatr Infect Dis 2010;17:137–147.

8. Sung JY, Kim JH, Yang MA, Kim SH, Eun BW, Lee J, et al. Usefulness of Interferon-γ measurement following stimulation of tuberculosis-specific antigens for diagnosis of latent tuberculosis infection in children exposed to pulmonary tuberculosis. Korean J Pediatr Infect Dis 2008;15:51–57.

9. Kim YJ, Lee JS. Comparison of interferon-gamma assays with the tuberculin skin test in children. Pediatr Allergy Respir Dis 2010;20:10–16.
10. Dogra S, Narang P, Mendiratta DK, Chaturvedi P, Reingold AL, Colford JM Jr, et al. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect 2007;54:267–276.


11. Lalvani A, Millington KA. T cell-based diagnosis of childhood tuberculosis infection. Curr Opin Infect Dis 2007;20:264–271.


12. Tieu HV, Suntarattiwong P, Puthanakit T, Chotpitayasunondh T, Chokephaibulkit K, Sirivichayakul S, et al. Comparing interferon-gamma release assays to tuberculin skin test in Thai children with tuberculosis exposure. PLoS One 2014;9:e105003



13. Lewinsohn DA, Zalwango S, Stein CM, Mayanja-Kizza H, Okwera A, Boom WH, et al. Whole blood interferon-gamma responses to mycobacterium tuberculosis antigens in young household contacts of persons with tuberculosis in Uganda. PLoS One 2008;3:e3407



14. Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, Hatherill M, Moyo S, Hanekom W, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 2011;30:694–700.


15. Garazzino S, Galli L, Chiappini E, Pinon M, Bergamini BM, Cazzato S, et al. Performance of interferon-γ release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J 2014;33:e226–e231.


16. Chiappini E, Bonsignori F, Accetta G, Boddi V, Galli L, Biggeri A, et al. Interferon-γ release assays for the diagnosis of Mycobacterium tuberculosis infection in children: a literature review. Int J Immunopathol Pharmacol 2012;25:335–343.


17. Pavic I, Topic RZ, Raos M, Aberle N, Dodig S. Interferon-γ release assay for the diagnosis of latent tuberculosis in children younger than 5 years of age. Pediatr Infect Dis J 2011;30:866–870.


18. Starke JR. Committee On Infectious Diseases. Interferon-γ release assays for diagnosis of tuberculosis infection and disease in children. Pediatrics 2014;134:e1763–e1773.


19. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006;10:1192–1204.

20. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010;59(RR-5): 1–25.
21. Howley MM, Painter JA, Katz DJ, Graviss EA, Reves R, Beavers SF, et al. Evaluation of QuantiFERON-TB gold in-tube and tuberculin skin tests among immigrant children being screened for latent tuberculosis infection. Pediatr Infect Dis J 2015;34:35–39.



22. Haustein T, Ridout DA, Hartley JC, Thaker U, Shingadia D, Klein NJ, et al. The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr Infect Dis J 2009;28:669–673.


23. Ferrara G, Losi M, D'Amico R, Roversi P, Piro R, Meacci M, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 2006;367:1328–1334.


24. Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax 2006;61:616–620.



25. Smart JM, Kemp AS. Ontogeny of T-helper 1 and T-helper 2 cytokine production in childhood. Pediatr Allergy Immunol 2001;12:181–187.


26. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000;161(4 Pt 1): 1376–1395.


Fig. 1
Interferon-γ released in response to nil antigen (A), tuberculosis antigen (B), and phytohemagglutinin (C) according to age.
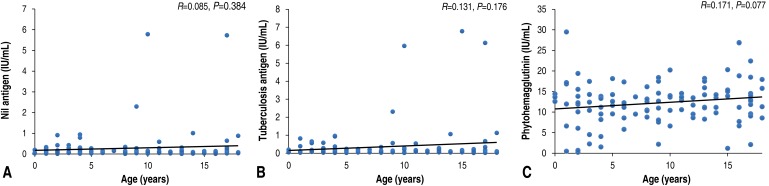
Fig. 2
Trends of interferon-γ release in serial IGRA testing. TB, tuberculosis; PHA, phytohemagglutinin; IGRA, interferon-γ releasing assay; TST, tuberculin skin test; PP, subject with positive results in both IGRA and TST; NP, subject with negative IGRA and positive TST results; NN, subject with negative results in both IGRA and TST.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


