Introduction
Preterm birth rates have increased globally, and according to a recent estimate, preterm births account for 10.6% of live births [
1]. In Korea, the survival rate of infants with very low birth weight more than doubled, increasing from 38.3% in the 1980s to 84.8% in 2014, while that of infants with extremely low birth weight increased more than fivefold, from 12.3% in the 1980s to 69.6% in 2014 [
2]. Therefore, there is a growing emphasis on the healthy growth of preterm infants beyond their survival.
Lung volume and function reach the maximum values in the late 20s and, then decline steadily with age. However, in patients who experienced an early lung injury or maldevelopment during infancy, respiratory symptoms may appear earlier, which can lead to reduced peak lung growth [
3]. Several cohorts of patients with preterm lung growth have been established to track the respiratory functions of prematurity survivors. Impaired pulmonary function, predominantly obstructive patterns, or structural alteration of the lungs have been reported in cohorts of very preterm- or extremely preterm-born children aged 5–11 years in the US, UK, Australia, and Italy [
4-
8].
Our recent study, using large-scale nationwide claims data, revealed that bronchopulmonary dysplasia (BPD) is associated with high respiratory morbidity in terms of rehospitalization rates, intensive care rates, and childhood asthma prevalence in recent survivors with respiratory distress syndrome (RDS) [
9]. However, considering the nature of the claims data, it was difficult to accurately determine the effects of prematurity and BPD associated with respiratory morbidity. Therefore, the impact of BPD on pulmonary functional and structural changes in school-aged preterm-born, surfactant-treated individuals needed to be investigated. In this context,we established the LONGitudinal cohort study of the population born preTERM (LONGTERM) to investigate serial lung development in preterm-born children from preschool age to adulthood; to the best of our knowledge, this is the first such study in Korea. In this interim data analysis from the LONGTERM cohort, we reported the functional and structural statuses of the participants. We also investigated whether BPD and other perinatal factors are associated with abnormal function in school-aged preterm-born children.
Methods
1. Participants
Children aged ≥5 years who were born prematurely, admitted to the Severance or Gangnam Severance Hospital neonatal intensive care units (NICU), and treated with pulmonary surfactants owing to RDS between 2005 and 2015 were recruited into the LONGTERM cohort. Patients who died, had congenital cardiopulmonary anomalies or neurologic impairments that made it challenging to perform pulmonary function tests (PFTs), or whose personal information could not be obtained were excluded. Among the preterm-born children with RDS (N=1,207), 57.8% (N=700) were eligible for enrollment in the LONGTERM cohort. We contacted 353 patients between November 2019 and August 2021, of whom 150 (48.1%) completed their visits; these patients’ data were analyzed (
Supplementary Fig. 1). The participants were then classified into 3 groups: non/mild, moderate, and severe BPD. This study was conducted in accordance with the principles of the Declaration of Helsinki. All participants and their parents were fully informed of the purpose and procedures of the study, and the parents provided their written informed consent before enrollment. The Institutional Review Board (IRB) of Severance Hospital (IRB No. 4-2019-0914) and Gangnam Severance hospital (IRB No. 3-2020-0195) reviewed and approved the study protocol.
2. Study design
Maternal and neonatal data were collected from the medical records of each hospital. Prior and current respiratory health status, environmental exposures, parental history of allergic diseases, and socioeconomic status data were obtained through questionnaires that were answered by parents. All participants underwent PFTs. Chest computed tomography (CT) and laboratory studies were performed after obtaining additional consent from the parents and children.
3. Definition of BPD
BPD was defined as at least 28 days of supplemental oxygen requirement and was graded as mild, moderate, or severe, according to the definitions of National Institutes of Health criteria proposed by Jobe and Bancalari [
10] More specifically, the severity of BPD was graded according to the respiratory support at 36 weeks postmenstrual age: mild BPD if room air was sufficient, moderate BPD if <30% oxygen was required, severe BPD if ≥30% oxygen or positive pressure was required.
4. Demographics
Maternal and neonatal data; gestational age, birth weight, sex, small for gestational age, delivery mode, twin birth, antenatal/postnatal steroid use, maternal risk factors (pregnancy-induced hypertension, chorioamnionitis, oligohydramnios, premature rupture of membrane), use of surfactant, the duration and mode of respiratory support, and comorbidities were collected from medical records. The height and weight of the patients were measured at the time of the visit for the PFT.
5. Asymptomatic patients
Based on medical records and questionnaire, participants were classified as “asymptomatic” when they fulfilled the following requirements: (1) no regular respiratory check-ups; (2) absence of subjective respiratory symptoms (e.g., cough, sputum, and dyspnea); and (3) no significant lower respiratory tract infections (LRIs) in the past year. Significant LRI was defined as an infection requiring medication for >1 week or hospital admission within the past 1 year.
6. Pulmonary function test
All tests were performed per the American Thoracic Society guidelines [
11]. Forced expiratory volume in 1 second (FEV
1), forced vital capacity (FVC), FEV
1/FVC ratio, and forced midexpiratory flow (FEF
25%–75%) were measured before and after inhalation of a bronchodilator. All parameters were calibrated according to age, sex, height, weight, and ethnicity [
12]. Estimates were obtained using the reference values of the Global Lung Function Institute (GLI) [
13]. A z score ≤-1.64 was considered below the lower limit of normal (LLN). Bronchodilator response (BDR) was considered positive compared with baseline data when FEV
1 increased by ≥12% after inhaling salbutamol compared with baseline data.
7. Chest CT
A pediatric radiologist with 18 years of experience in pediatric chest CT performed the semiquantitative assessment of disease severity using CT images. We modified a previously reported scoring system based on lobes rather than lung segments [
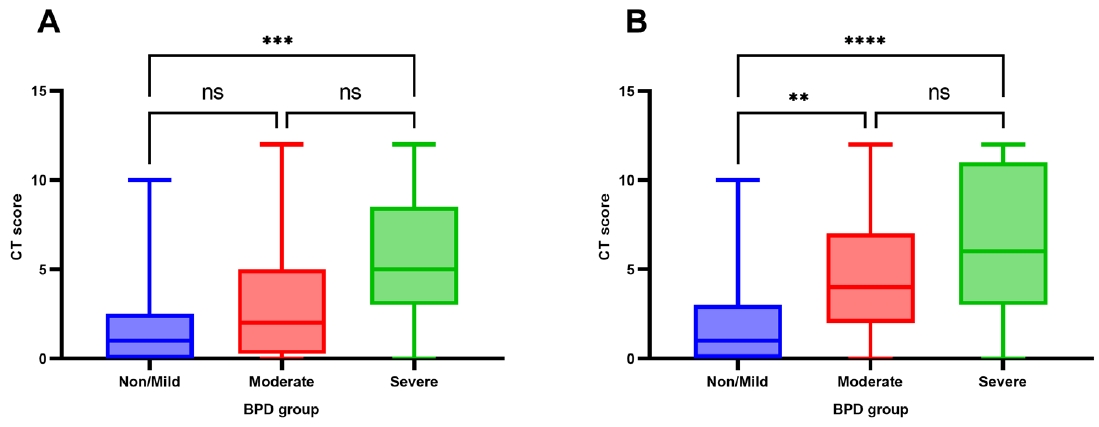
14]. The presence (score 1) or absence (score 0) of lung lesions, including 2 categories of hyperaeration and parenchymal lesions, was evaluated for each lung lobe. The left lingular segment was considered one lobe. The hyperaeration score (range, 0–6) referred to the number of lobes showing areas of reduced lung attenuation, mosaic attenuation pattern, or bullas/blebs. The parenchymal lesion score (range, 0–6) referred to the number of lobes with linear lesions, segmental atelectasis, consolidation, or architectural distortion. The sum of these 2 scores was used as the CT severity score assigned to each patient. CT severity was compared based on the severity of BPD (non/mild, moderate, or severe BPD) and PFT results (below the LLN or above the LLN for each parameter).
8. Laboratory studies
General health information, including leukocyte counts with differential; platelet counts; and hemoglobin, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, and C-reactive protein level measurements, was obtained for each participant from their laboratory studies. Total serum immunoglobulin E (IgE) levels were measured using the ImmunoCAP assay (Thermo Fisher Scientific, Uppsala, Sweden), and specific IgE levels were measured using a multiple antigen simultaneous test immunoblot assay for 61 inhalants (AdvanSure Alloscreen; LG Life Science, Seoul, Korea). Atopy was defined as a specific IgE level of ≥2 against >1 allergen or a total IgE level of 150 IU/mL.
9. Statistical analysis
Data were analyzed using R (ver. 4.1.1; R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). Categorical variables are presented as frequency (%)for each group and continuous variables are presented as the median with interquartile range (IQR). PFT and CT score data are presented as median (ranges). The values were compared among the 3 BPD groups using Fisher exact test for the categorical variables and the Kruskal-Wallis test, followed by Bonferroni post hoc analysis for the continuous variables. The 2-sample t test was used to compare values between the groups with PFT results below or above the LLN. The values of asymptomatic and symptomatic patients were compared using the Mann-Whitney U test for the continuous variables and Fisher exact test for the categorical variables. Univariate and multivariate logistic regression analyses were used to analyze the influence of independent perinatal risk factors on PFT results. Univariate and multivariate quantile (median) regression analyses were used to assess the influence of independent perinatal factors on chest CT scores. Variables that showed statistical significance in the univariate analysis were selected as covariates for the multivariate regression. Statistical significance was set at P<0.05.
Discussion
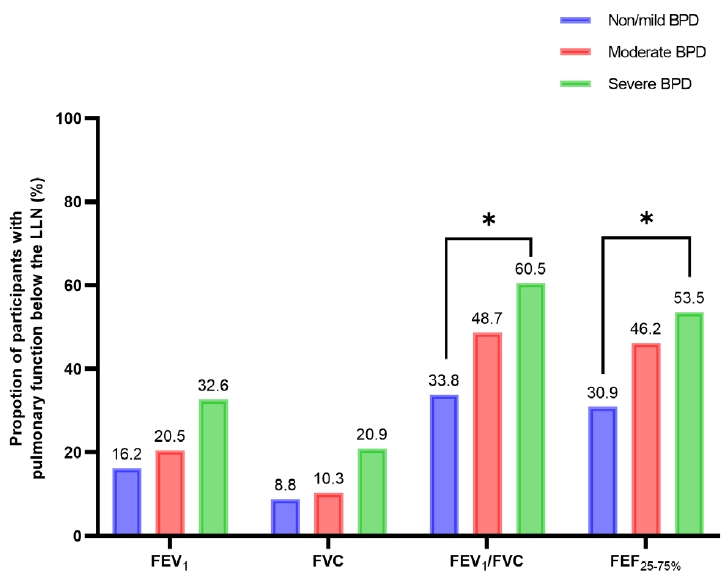
To our knowledge, this is the first study to investigate objective pulmonary functional and structural changes in preterm children using a longitudinal cohort in Korea. This study demonstrated that school-aged children with severe BPD had significantly impaired pulmonary function in terms of FEV1/FVC and FEF25%–75% compared with those with moderate or non/mild BPD. The median z score or %pred of FEV1 and FVC in the severe BPD group were lower than those of the non/mild and moderate BPD groups; however, they still fell within the normal range. Chest CT showed structural lung abnormalities in the severe BPD group. The presence of abnormalities on chest CT was also significantly higher in the severe BPD group than the non/mild BPD group in asymptomatic patients. However, after adjusting for multiple perinatal factors, severe BPD was not independently associated with poor pulmonary function. Prenatal oligohydramnios and a longer duration of ventilator care were strongly associated with a risk of FEV1/FVC below the LLN, and a higher chest CT score.
Data from previous cohort studies of pulmonary function in preterm-born children showed impaired lung function at 8–12 years of age, mainly in the form of airflow obstruction (decreased FEV
1), which was seen in children with BPD and, to a lesser degree, in children without BPD. Levin et al. [
7] explained that this obstructive pattern suggests dysanaptic or differential growth of the airways and lung parenchyma. Narayanan et al. [
15] reported lower z-FEV
1 in preterm infants born at <32 weeks of gestation, more dominantly in those with BPD, and similar alveolarization in preterm and term infants through a helium-3 magnetic resonance scan study. Our data also demonstrated an obstructive pattern of pulmonary function, although the FEV
1 trend was relatively normal, and the FEV
1/FVC was significantly reduced, demonstrating the airflow limitation is the main pathophysiology of these population.
Notably, impaired pulmonary function was found even in asymptomatic patients. Among patients without subjective respiratory symptoms, 36% and 30% had FEV1/FVC and FEF25%–75% below the LLN, respectively. Moreover, one-third of the patients with non/mild BPD had FEV1/FVC and FEF25%–75% below the LLN. These findings indicate the presence of significantly impaired pulmonary function, even in preterm children without oxygen dependency at a postmenstrual age of 36 weeks and in asymptomatic preterm- born children.
Arroyas et al. [
16] reported that preterm birth at <32 weeks of gestation was an independent risk factor for asthma in adolescence. In the present study, asthma was diagnosed more frequently in the severe BPD group than in the other groups. However, the proportion of participants with BDR ≥12% and allergic sensitization did not differ among the groups. This can be attributed to a fixed or structural component (dysanpasis) of BPD, although the obstructive component of BPD may also be due to airway inflammation and smooth muscle hypertrophy, which are responsive to standard asthma management [
17].
Structural analysis of preterm-born children has been performed in a few single-center cohort studies. One study from Australia showed parenchymal structural changes using chest CT at age 9–11 years; these resulted from significant abnormalities in 92% of children born at ≤32 weeks of gestation [
18]. In the same study, air trapping observed on CT scans was associated with an early gestational age and increased continuous positive airway pressure duration. However, lung volumes were generally not different between preterm children with and without BPD. Extensive remodeling of the lungs leads to changes in lung architecture with regional heterogeneity, showing features such as lung hyperinflation and abnormal parenchymal lesions [
19]. Our data showed that the semi-quantitative chest CT score correlated with BPD severity and low pulmonary function (FEV
1, FEV
1/FVC, and FEF
25%–75%).
BPD is often defined as a developmental disorder, with its pathogenesis being linked to underdeveloped lungs, inflammation, barotrauma or volutrauma due to mechanical ventilation, and oxidative stress due to respiratory treatment of immature lungs [
20]. The lungs of prematurity survivors with and without BPD show abnormal parenchymal and vascular growth, with frequent respiratory infections in the first 2 years of life [
21]. In addition, some of these patients fail to reach optimal peak lung function in early adulthood. Furthermore, many studies have shown abnormalities in lung function and structures in patients with BPD; these persist throughout childhood, adolescence, and young adulthood in patients with BPD [
19]. Moreover, studies have shown mixed results regarding whether children with a history of very low birth weight and BPD exhibited any difference in lung function compared with those with a history of very low birth weight without BPD [
3]. However, as these studies used different criteria for gestational age and birth weight and different definitions of BPD, it is difficult to compare the results. Reportedly, 20%–25% of patients with chronic obstructive pulmonary disease (COPD) have never smoked [
22,
23]. Early life events are also associated with the development of chronic airway obstruction [
24]. Notably, few longitudinal studies have been conducted after early adulthood; however, preterm infants may be at an increased risk of developing COPD later in life [
25].
According to our multivariate analysis, oligohydramnios and duration of ventilator care, but not BPD, were independently associated with both low lung function and abnormal structure. This is consistent with the conclusions of other recent large-scale studies. Hart et al. [
26] suggested that gestational age and intrauterine growth restriction, but not BPD, were significantly associated with poor lung function in preterm-born children aged 7–12 years. Developmental restriction of alveolar and vascular structures in utero and further insults after birth might be the underlying pathogeneses of chronic lung disease in preterm infants. These results also disprove that traditional BPD diagnostic criteria cannot predict long-term respiratory prognosis directly.
New etiologies for COPD were recently proposed, and COPD due to abnormal lung development was reclassified as COPD-D (COPD due to abnormal lung development was reclassified as COPD-D) [
27]. Through longitudinal follow-ups of preterm-born children through adulthood, functional and structural changes will be serially monitored, allowing individualized therapeutic or preventive strategies for impaired lung function to be provided. In addition, we plan to explore early prediction models for risk stratification in preterm infants by combining perinatal and environmental data related to pulmonary function with imaging data from a larger number of participants. Furthermore, assessing more recent patients is essential when evaluating the longterm outcomes of the recently introduced ventilator strategy (noninvasive ventilator care from early life) and enhanced general supportive care of preterm infants.
Our study has some limitations. First, children whose caregivers were concerned about their respiratory health may have been more likely to participate in the study. This may have led to a selection bias,particularly among asymptomatic individuals. Second, some patients with severely declining respiratory function may have been less likely to be included in our study cohort because we excluded children who could not undergo PFTs owing to neurologic impairment. However, patients with severe BPD had significantly lower body weight for their age at the time of PFT compared with those om the other 2 groups; therefore, there is possibility that being underweight may have influenced the PFT results. Third, we used the internationally accepted GLI scores as the standard reference for our cohort instead of a control group. Finally, the extent of the chest CT findings is likely to be limited by the insensitivity of the scoring method because it does not account for multiple presentations within the same lobe, as observed in previous studies.
In conclusion, preterm-born children, especially those with severe BPD, are at a high risk of pulmonary functional and structural abnormalities at school age. Of note, oligohydramnios and the duration of mechanical ventilation after birth can be prenatal and postnatal independent risk factors for low lung function and abnormal structure, respectively. Therefore, longitudinal follow-ups and screening for lung function and adequate interventions are warranted in preterm infants, including those who are asymptomatic.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Download Citation
Download Citation


