Introduction
Intraventricular hemorrhaging (IVH) is a condition in which a germinal matrix hemorrhage ruptures through the ependyma into the lateral ventricle; it occurs mainly in premature infants. The risk of IVH increases with decreasing immaturity1). Consequently, the recent improvements toward the survival of very preterm infants through advances in neonatal intensive care medicine have resulted in an increased number of infants being at a high risk of developing IVH2). A direct correlation exists between the extent of immaturity and the severity of IVH3). More than 50% of preterm infants with severe IVH (grade>3) die or develop posthemorrhagic hydrocephalus (PHH), which requires shunt surgery in up to 70% of the cases4). Brain white-matter damage, which is induced by severe IVH, is exacerbated by PHH and results in increased mortality and long-term neurological morbidities such as seizures, cerebral palsy, and developmental retardation in the survivors5,6,7). Currently, no effective therapies exist to ameliorate brain injury and prevent PHH development after severe IVH. Thus, the development of new therapeutic modalities to improve the prognosis of severe IVH in preterm infants is an urgent requirement.
Pathogenesis of PHH after severe IVH
The germinal matrix is a highly vascularized structure located along the lateral ventricle. Its intrinsic fragility is related to the immaturity of the blood-brain barrier, and it contributes to primary bleeding into the germinal matrix. Multiple risk factors such as vaginal delivery, low Apgar score, respiratory-distress syndrome, pneumothorax, hypoxia, hypercapnia, and infection can be attributed to the disturbance in the cerebral blood flow and to the induction of IVH in preterm infants. After bleeding from the germinal matrix into the cerebral ventricles, subsequent hemolysis in the intraventricular space elevates the concentration of extracellular hemoglobin. The ensuing heme degradation increases the concentrations of bilirubin, carbon monoxide, and free iron8) in the cerebrospinal fluid (CSF). Cell-free hemoglobin is known to initiate proinflammation, chemotaxis, and apoptosis in intracranial hemorrhaging9,10). Extravasated blood in the CSF activates an inflammatory response in the microvascular barrier and results in the dysfunction of arachnoid granulations, which reduces resorption of the CSF. This imbalance in CSF production and resorption results in the retention of CSF and progressive hydrocephalus. In newborn infants, hydrocephalus itself causes brain-parenchymal injury through various mechanisms, including increased intracranial pressure and the resultant decrease in cerebral perfusion, iron-induced free radical damage, and inflammatory cytokine levels11,12,13,14,15).
Neonatal animal models of IVH and hydrocephalus
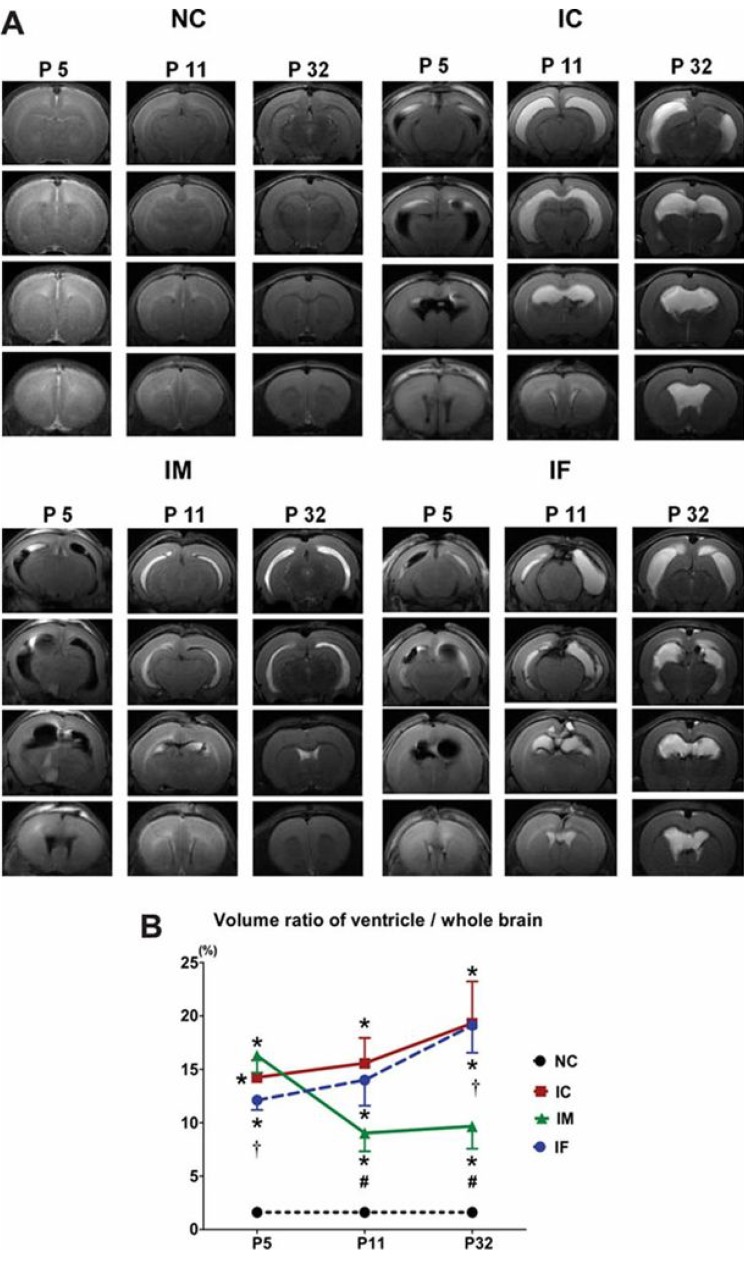
Development of an appropriate animal model that simulates severe IVH in premature infants is essential for understanding its pathophysiology and for testing the efficacy of new therapeutic strategies. However, no single model is suitable for studying all the aspects of brain injury. Some animal models of neonatal IVH were induced via the direct injection of blood into the brain of newborn animals16,17,18,19,20). A rodent model was induced by administering a bilateral injection of 80 µL blood into the cerebral ventricles16), and another model was induced by administering a blood injection into the periventricular region17). However, only 15%-65% of these models developed significant PHH16,17,21). In another model, germinal matrix hemorrhage (GMH) was developed by injecting clostridial collagenase into the germinal matrix, but no ventricular dilatation was observed22). Another GMH-IVH animal model precipitated GMH by intraperitoneal injection of glycerol into preterm rabbits delivered at 29 days gestation (full term is 32 days)23). Only 39% of this rabbit model developed severe IVH that was clinically relevant to grade 3 or 4 IVH-the main cause of PHH in preterm infants23). We recently reported a modeling method in which direct injection of dam's blood (200 µL) into the cerebral ventricles of postnatal day (P) 4 rat pups developed IVH animal models24). With this method, 100% of the rats developed severe IVH (compatible with grade 3 or 4 IVH), as confirmed via brain magnetic resonance images (MRIs) on the first day after modeling. About 85% of these pups subsequently developed progressive PHH, which lasted for more than 4 weeks after the modeling24) (Fig. 1). In addition, as the rodent brains at birth and at P10 are compatible with those at 24- and 40-weeks' gestation, respectively, the rodent brain at P4 should represent the developmental state of the brain in preterm infants25). About 4 weeks after IVH induction, at P4, the rats showed significantly impaired sensorimotor functions and augmented cell death, inflammation, and delayed myelination in the brain tissue with significantly upregulated inflammatory cytokines in the CSF24). This observation suggested the replication of the pathogenesis of IVH in preterm infants in that hemolysis from the extravasated blood activated inflammation in the subarachnoid space, resulting in hydrocephalus and subsequent pressure injury to the brain tissue.
Attempt to downregulate inflammation and reduce PHH after severe IVH
Research on newborn IVH has shown that transforming growth factor (TGF)-β is associated with the accumulation of extracellular matrix proteins and collagen, which probably interferes with the CSF flow26,27,28). However, several different TGF-β blocking agents failed to stop the progression of ventricular dilatation and did not improve the neuromotor performance in an IVH neonatal rat model19,20). Increasing evidence suggests that inflammatory cytokines with activated macrophages are detrimental to neural cells. Inflammatory mechanisms appear to be the key factors in secondary brain injury, such as neuronal-cell death and reactive gliosis after intracerebral hemorrhaging29,30,31). A study involving the newborn rabbit IVH model showed that the inhibition of cycloxygenase-2 in the inflammatory cascade, which is induced by IVH, alleviated neurological impairment and delayed myelination and reactive gliosis32). Another study using this model established that the inhibition of bone morphogenic protein restored oligodendrocyte maturation, myelination, astrocyte morphology, and motor function33). These therapeutic strategies did not reduce the size of enlarged cerebral ventricles after IVH33); however, these reports suggest that modulating only one factor is not sufficient to improve PHH and that a multifaceted therapeutic agent is needed.
Therapeutic potential of MSCs for severe IVH
Stem-cell therapy has shown promising results in a number of brain injury or disease models34,35,36,37). Among the therapeutic sources, mesenchymal stem cells (MSCs) are well known for their anti-inflammatory property. In a mixed-lymphocyte reaction, MSCs reduced T-lymphocyte proliferation38,39), probably due to their lack of major histocompatibility-complex class II antigens, which prevent the development of a graft-versus-host response40). In addition, MSCs have been known to shift the inflammatory response by polarizing the cytokine profile of T-cell subsets to an anti-inflammatory phenotype. This low-immunogenic property of MSCs is believed to be related to the secretion of various factors that create an immunosuppressive environment41,42). MSCs decrease TNF-α, interleukin (IL) 12, and interferon-γ production and increase IL-10 production in dendritic cells43). Besides the anti-inflammatory effects, various neurotropic factors secreted from MSCs promote angiogenesis, myelination, and rewiring of neurons and reduce gliosis and cell death. Thus, in a number of disease models, various paracrine factors such as numerous anti-inflammatory cytokines and tropic factors secreted from MSCs display potent anti-inflammatory efficacy by restoring brain injuries. Until date, single agents such as decorin, colchicine, TGF-β blocker, nonsteroidal anti-inflammatory drugs, and bone-morphogenic protein inhibitor have not proven effective in reducing the brain damage and PHH after severe IVH24). Recently, we used various animal disease models to demonstrate the beneficial effects of MSC transplantation, including antiapoptotic, anti-inflammatory, antifibrotic, antioxidative paracrine, and regenerative effects24,44,45,46,47,48). Considering its multiple therapeutic efficacies, MSCs, rather than single agents, are potential candidates for therapies aimed at improving the outcome of severe IVH in preterm infants.
Remaining challenges in MSC transplantation for severe IVH
We demonstrated that intraventricular transplantation of MSCs significantly attenuated inflammatory cytokines of the CSF and brain tissue (Fig. 2) and prevented the development of PHH after severe IVH (Fig. 3) in P4 newborn rats24). MSC transplantation significantly improved brain apoptosis and reactive gliosis and reduced myelin basic protein and abnormal sensorimotor functions in severe IVH. The neuroprotective mechanism appeared to be mediated by the paracrine anti-inflammatory effects rather than the regeneration of transplanted MSCs. Overall, the findings of the present study support the therapeutic potential of MSC transplantation in improving the prognosis of the serious complications of preterm birth, i.e., severe IVH. However, several hurdles remain, before we can achieve safe clinical transplantation of MSCs in preterm infants with severe IVH. Techniques must be developed and standardized for defining, isolating, and expanding MSCs. Although our previous study demonstrated the therapeutic efficacy of MSC transplantation, further issues remain to be addressed, such as the optimal dose, the route, and the timing of MSC transplantation. Safe clinical use of MSC transplantation to treat brain damage and prevent PHH in preterm infants with severe IVH requires further delineation of the neuroprotective mechanism of MSC and the assessment of the short- and long-term safety and efficacy of MSC transplantation.
Conclusions
Our recent observation that MSCs transplantation significantly attenuated the PHH and brain injury after severe IVH in the newborn rats holds future promise of stem cell therapy as novel therapeutic modality for treating the currently intractable severe IVH in the premature infants. However, for safe and successful clinical translation of stem cell therapy, further preclinical studies including the determination of optimal route, dose and timing, and the assessment of the short-term and long-term efficacy and safety of cell-based therapies are warranted.





 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


