Introduction
Pyogenic liver abscess (PLA) in children is rare in developed countries and has been frequently associated with disorders of granulocyte function and ascending pylephlebitis, complicating previous abdominal infection1). The global reported incidence for PLA is variable, ranging from 3 to 25 per 100,000 pediatric hospital admissions2-4). PLA is a potentially life threatening condition, however, the mortality rate has been decreased due to advances in imaging technology, drainage techniques, and more effective antibiotic treatment3). Escherichia coli used to be the most common organism causing PLA, but recently, Klebsiella pneumoniae has emerged as a leading pathogen in Korea, the Unite States, and other countries5-10). K. pneumoniae liver abscess (KLA) can lead to serious complications, including bacteremia and extrahepatic abscesses11). This case describes an immunocompetent 12-year-old boy presenting with KLA without underlying disease.
Case report
A 12-year-old boy was admitted to the Department of Pediatrics, Kangbuk Samsung Hospital, with a 4-day history of fever and headache. The day before admission, he vomited several times. On review of systems, the patient noted fatigue, headache, nausea, and vomiting, but denied any other symptoms, such as abdominal pain, diarrhea or visual change. He has been healthy without admission history or frequent infections. Family history was not remarkable. He did not have history of traveling, tick bites, or sick contacts during the last 6 months. His height was 158 cm (50th to 75th percentile) and weight was 45 kg (50th to 75th percentile). His initial vital signs included a temperature of 39℃, a heart rate of 126 beats/min, blood pressure of 118/74 mmHg, and respiratory rate of 20 breaths/min. He was alert. The scleras were not icteric and the neck was supple. The lungs were clear bilaterally, with no audible murmur on cardiac auscultation. The abdomen was soft and nontender, with no hepatosplenomegaly. The neurologic examination was unremarkable. The complete blood cell counts on the day of admission were hemoglobin (Hb), 12.0 g/dL; hematocrit (Hct), 35.1%; platelet, 179,000/mm3; and white blood cell (WBC), 15,400/mm3 (neutrophil, 85%; lymphocyte, 4%; monocyte, 11%). The blood chemistry showed glucose, 95 mg/dL (reference range, 60 to 100 mg/dL); aspartate aminotransferase (AST), 113 IU/L (reference range, 15 to 40 IU/L); alanine aminotransferase (ALT), 94 IU/L (reference range, 5 to 45 IU/L); lactate dehydrogenase, 605 IU/L (reference range, 120 to 330 IU/L); total bilirubin, 1.18 mg/dL (reference range, 0.2 to 1.3 mg/dL); total protein, 7.1 g/dL (reference range, 6.4 to 8.1 g/dL); albumin, 3.8 g/dL (reference range, 4.0 to 5.3 g/dL); alkaline phosphatase, 142 IU/L (reference range, 116 to 483 IU/L); γ-Glutamyl transpeptidase, 21 U/L (reference range, 25 to 24 U/L); and C-reactive protein (CRP), 22.05 mg/dL (reference range, <0.18 mg/dL). Coagulation panel demonstrated an international normalized ratio of 1.10 (reference rage, 0.89 to 1.10), prothrombin time of 12 seconds (reference range, 10 to 12 seconds), and activated partial thromboplastin time of 27 seconds (reference range, 26 to 37 seconds). The patient was negative for hepatitis B surface antigen and antibodies to hepatitis A and C virus. Abnormal findings were not observed in either the cerebrospinal fluid (CSF) study or the urinalysis test. Cultures of blood, urine and CSF were negative. The initial chest and abdomen X-ray had no remarkable findings.
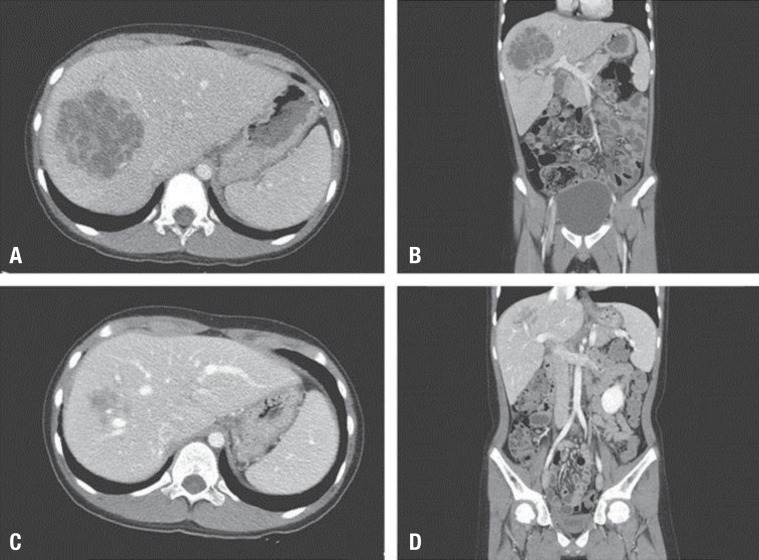
Five hours after the admission, he complained of right upper quadrant abdominal pain abruptly. Right upper quadrant tenderness was noted, but rebound tenderness was not. Due to abnormalities of liver function tests and abdominal pain, emerency abdominal computed tomography (CT) scan was ordered; it demonstrated 7.6 cm×9 cm×7 cm sized ill-defined multiply septated low density lesions in segments VII and VIII of the liver, consistent with hepatic abscess (Fig. 1A, B). Hepatoduodenal ligament and lymph nodes were enlarged and these findings were regarded as reactive hyperplasia associated with the hepatic abscess. There were no remarkable findings in the gastrointestinal tract including appendix and other solid organ. Venous thrombosis was not seen in abdominal CT. The portal vein, hepatic vein and its branch were patent. The patient was treated empirically with parenteral antibiotics (metronidazole, 30 mg/kg/day; ceftriaxone, 100 mg/kg/day; amikacin, 22.5 mg/kg/day) and underwent ultrasound-guided percutaneous catheter drainage (PCD) of the liver abscess. Eight-French multiple-sidehole pigtail catheter were inserted into the abscess cavity and about 30 mL of thick dark-yellowish pus was drained. The procedure was performed under local anesthesia with the patient in supine position. Conscious sedation was not used. Drainage of the abscess yielded purulent material that grew K. pneumonia sensitive to all tested antibiotics, except for ampicillin and piperacillin. Amoeba antibody was negative. To evaluate the immune status of the patient, serum immunoglobulins (Ig), complements (C), and lymphocyte subsets were measured and the dihydrorhodamine (DHR) test was performed. Serum IgG (1,180 mg/dL), IgA (331.1 mg/dL), IgM (72.5 mg/dL), C3 (86.2 mg/dL), C4 (14.89 mg/dL), total complement activity (CH50 47.7 U/mL), and lymphocyte subsets, including CD3 positive T-cells (87%, 2,514/µL), CD4 positive T-cells (T4, 44%, 1,271/µL), CD8 positive T-cells (T8, 31%, 896/µL), T4/T8 ratio (1.42), CD19 positive B-cells (7%, 202/µL), and CD3 negative and CD16 and CD56 positive natural killer cells (6%, 173/µL), were all in the normal range for his age. No abnormal findings were observed in the DHR test.
On hospital day 11, the fever subsided. Also his headache, and right upper quadrant abdominal pain were improved. The laboratory findings were Hb, 11.8 g/dL; Hct, 34.8%; WBC, 10,600/mm3 (lymphocyte, 27%; neutrophil, 64%; monocyte, 7%; eosinophil, 2%); platelet, 495,000/mm3; CRP, 3.81 mg/dL; AST, 24 IU/L; and ALT, 8 IU/L. On hospital day 12, a follow-up abdominal CT scan revealed a slightly decreased, but residual hepatic abscess (5.9 cm×6.1 cm×5.5 cm) in the right lobe and bilateral pleural effusion with passive atelectasis. Pus (40 to 120 mL daily) was drained initially, but the rate of drainage gradually decreased to less than 10 mL per day over 3 weeks and drainage material became serous. On hospital day 20, a right upper quadrant ultrasound showed that the size of the liquefied abscess cavity had reduced to 3 cm. The percutaneous catheter was removed on hospital day 21 because the amount of drainage was scanty. The total amount of pus drained from abscess cavity was 370 mL for 20 days. On hospital day 25, subsequent ultrasound demonstrated a significant reduction in size of the abscess. Laboratory findings on hospital day 28 were improved as follows: WBC, 5,900/mm3 (neutrophil 48.9%); CRP, below 0.5 mg/dL; AST, 34 IU/L; and ALT, 10 IU/L. The patient was discharged on hospital day 29 and was switched to oral forms of treatment. The oral antibiotic, cefixime, was administered for 14 days. Two weeks after discharge, the third follow-up abdominal CT scan was performed in an outpatient setting: it revealed that the abscess of the right lobe was almost healed and the bilateral pleural effusions with passive atelectasis were disappeared (Fig. 1C, D). The patient is now under follow-up care and has remained well over the last five months.
Discussion
In Korea, E. coli and anaerobes were commonly isolated organisms from PLA patients, but recent studies showed that K. pneumoniae has become the most common cause for PLA5,6). Biliary disease is the leading cause of PLA, except for cases of KLA, which are more likely to be cryptogenic12). Yang et al.13) reported that 64% of KLAs were cryptogenic. We did an extensive work-up to try to identify an intraabdominal infection source that might explain PLA of our ptient; however, no such focus was found.
The clinical symptoms of patients with KLA are always non-specific, such as fatigue, anorexia and fever. Some patients may present more specific clinical clues, such as right upper quadrant pain and jaundice. Fever is the most common presenting symptom in 70-95% of the reported cases8,14). On the other hand, abdominal pain was only found in 58.3% of patients in the previous study7). Diagnosis of KLA is difficult without the aid of imaging. Therefore it is important to consider prompt imaging studies of the abdomen for early diagnosis of KLA, if the patient has symptoms that suggest the infection and lab findings such as abnormalities of liver function tests, high CRP and leukocytosis.
In addition to Korea, the recent increase in frequency of KLA has been described also in Taiwan and China7,14). The reports from non-Asian countries found that most of the cases of KLA were found in patients of Asian descent10,15). Diabetes mellitus is a known risk factor for developing KLA, and it appears to be a significant risk factor for complications including embolic events7,16). Some studies have described cholelithiasis, malignancy and prior intraabdominal surgery as risk factors for KLA, but those diseases much commonly found in polymicrobial PLAs than in KLA11,16).
Although it was not seen in the reported case here, bacteremia is very common in patients with a KLA and has been documented in 83-95% of KLA cases15,16). This differs from non-KLAs, in which bacteremia was found in only 50% of cases9). As a result, KLA often led to endorgan seeding and extrahepatic abscess. Distant abscesses have been reported, most commonly in the eye, but also in the lung, meninges, kidney, skin, brain, and bone16). Thrombophlebitis, disseminated intravascular coagulation, and acute renal failure are also reported as well known complications of KLA4,7,11,16).
In Korea, as K. pneumoniae has emerged as the most common organism cuasing PLA5,6), the physician should consider the possibility of K. pneumoniae when selecting antibiotics for hepatic abscesses and a combination of an aminoglycoside and either an extended spectrum beta-lactam, or a third generation cephalosporin is a recommended regimen15). The standard antibiotic regimen for liver abscesses, including ampicillin, can be ineffective against KLA because K. pneumoniae is usually resistant to ampicillin17). One previous study reported that they discovered a rate of 13% for resistance to piperacillin, as well as ampicillin7). In addition to the antibiotic therapy, PCD is the mainstay therapy for PLA. PCD has been known as an effective and safe treatment of PLA with a low rate of complications which include hemorrhage, perforation of hollow viscera, peritoneal spillage, catheter displacement or blockage, and sepsis18). Although complications of PCD are infrequent, the sequela can be serious therefore PCD should be performed under active monitoring of vital sign. Percutaneous transhepatic drainage of the pus collection can be helpful in determining the causative organisms and can shorten the duration of treatment19). Overall, mortality for KLA has ranged from 6-17%, with intravenous antibiotics and drainage7,11-13). Our patient required PCD with antibiotic therapy. The catheter placed in intrahepatic abscess was left for 3 weeks until abscess collapse. Parenteral antibiotics, including amikacin, ceftriaxone and metronidazole, were administered during his inpatient stay (29 days), followed by oral antibiotics for 2 weeks in the outpatient department.
Although KLA is an emerging worldwide problem, KLA usually occurs in the presence of previous abdominal infection, biliary tract disease, and immunocompromised status. KLA in healthy patients is uncommon and articles about KLA in adult without underlying diseases have been rarely published in developed countries including Korea20-22). Intriguingly, our reported case is an immunocompetent child without above risk factors, which is rather a rare phenomenon. To our knowledge, this is the first case of KLA in healthy children in Korea. This unusual case serves as a reminder that KLA may present in an immunocompetent child. The possibility of KLA should be suspected when the immunocompetent host without underlying disease presents the characteristics of a liver abscess. We reported this case to raise awareness of KAL in immunocompetent children among pediatrician and to review the diagnosis, risk factors, potential complications and the appropriate treatment of KLA.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


