Article Contents
| Clin Exp Pediatr > Volume 68(9); 2025 |
|
Abstract
Background
Purpose
Methods
Results
Footnotes
Fig. 1.
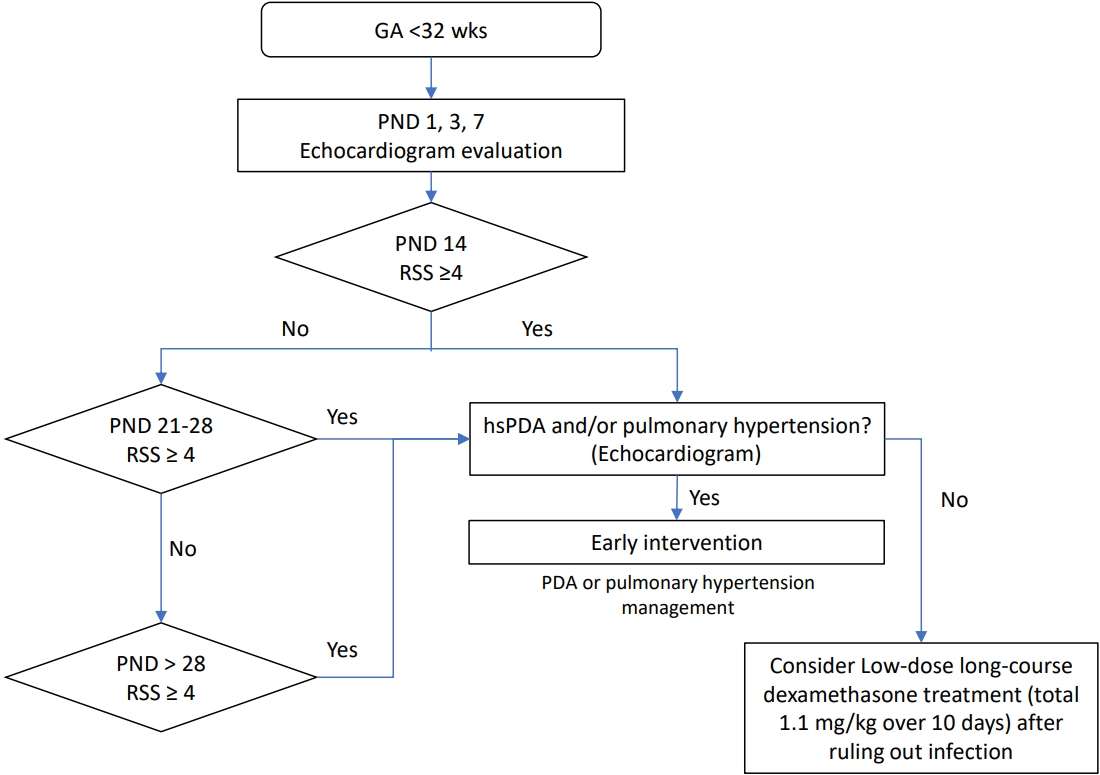
Table 1.
Values are presented as mean±standard deviation or number (%).
GA, gestational age; SGA, small for GA; DM, diabetes mellitus; PROM, premature rupture of membranes; hCAM, histological chorioamnionitis; PEEP, positive end-expiratory pressure; PPV, positive pressure ventilation; RDS, respiratory distress syndrome; INSURE, INtubation-SURfactant-Extubation; RSS, respiratory severity score; PND, postnatal day; HFOV, high-frequency oscillatory ventilator; NAVA, neurally adjusted ventilatory assist; PDA, patent ductus arteriosus; HTN, hypertension; NEC, necrotizing enterocolitis; MRI, magnetic resonance imaging; HTN, hypertension; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia.
Boldface indicates a statistically significant difference with P<0.05.
Table 2.
Table 3.
| Variable | Phase I (2010–2014) (n=108) | Phase II (2016–2022) (n=100) | aOR (95% CI)b) | P value |
|---|---|---|---|---|
| Respiratory outcomes | ||||
| Jensen grade | (n=99) | (n=96) | ||
| Grade 0 | 15/99 (15.2) | 29/96 (30.2) | 2.31 (1.10–4.83) | 0.024 |
| Grade 1 | 28/99 (28.3) | 28/96 (29.2) | 0.98 (0.50–1.89) | 0.941 |
| Grade 2 | 39/99 (39.4) | 30/96 (31.3) | 0.83 (0.44–1.56) | 0.559 |
| Grade 3 | 17/99 (17.2) | 9/96 (9.4) | 0.42 (0.17–1.08) | 0.064 |
| Respiratory support at discharge | ||||
| Nasal prong | 44/99 (44.4) | 37/95 (38.9) | 0.87 (0.47–1.60) | 0.644 |
| High flow nasal cannula | 0/99 (0) | 1/95 (1.1) | - | - |
| Tracheostomy | 3/99 (3.0) | 2/95 (2.1) | 0.45 (0.05–3.86) | 0.466 |
| Readmission within 12 months due to respiratory causes | 36/98 (36.7) | 35/95 (36.8) | 1.23 (0.66–2.31) | 0.512 |
| Neurodevelopmental outcomes | ||||
| Death or any NDIa) | 24/107 (22.4) | 17/100 (17.0) | 0.70 (0.34–1.45) | 0.337 |
| Any NDIa) | 17/96 (17.7) | 12/95 (12.6) | 0.72 (0.31–1.68) | 0.446 |
| Cerebral palsy | 14/96 (14.6) | 10/95 (10.5) | 0.72 (0.29–1.79) | 0.474 |
| Hearing loss | 5/96 (5.2) | 1/95 (1.1) | 0.26 (0.03–2.44) | 0.238 |
| Visual impairment | 0/97 (0) | 1/95 (1.1) | - | - |
| Death | 9/107 (8.4) | 5/100 (5.0) | 0.50 (0.14–1.71) | 0.266 |
Table 4.
| Variable | Phase I (2010–2014) (n=108) | Phase II (2016–2022) (n=100) | aOR (95% CI)b) | P value |
|---|---|---|---|---|
| Respiratory outcomes | ||||
| Jensen grade | ||||
| Grade 0 | 1/19 (5.3) | 2/33 (6.1) | 1.56 (0.12–19.79) | 0.730 |
| Grade 1 | 1/19 (5.3) | 11/33 (33.3) | 12.22 (1.19–125.90) | 0.035 |
| Grade 2 | 8/19 (42.1) | 13/33 (39.4) | 0.78 (0.22–2.78) | 0.705 |
| Grade 3 | 9/19 (47.4) | 7/33 (21.2) | 0.26 (0.07–1.00) | 0.050 |
| Respiratory support at discharge | ||||
| Nasal prong | 11/19 (57.9) | 19/32 (59.4) | 1.17 (0.34–4.04) | 0.801 |
| High flow nasal cannula | 0/19 (0) | 0/32 (0) | - | - |
| Tracheostomy | 3/19 (15.8) | 1/32 (3.1) | - | - |
| Readmission within 12 months due to respiratory causes | 11/18 (61.1) | 16/32 (50.0) | 0.86 (0.24–3.07) | 0.820 |
| Neurodevelopmental outcomes | ||||
| Death or any NDIa) | 4/19 (22.2) | 2/33 (6.1) | 0.28 (0.04–1.91) | 0.195 |
| Any NDIa) | 4/17 (23.5) | 1/32 (3.1) | 0.11 (0.01–1.19) | 0.069 |
| Cerebral palsy | 3/17 (17.6) | 1/32 (3.1) | 0.16 (0.01–1.93) | 0.149 |
| Hearing loss | 2/17 (11.8) | 0/32 (0) | - | - |
| Visual impairment | 0/18 (0) | 0/32 (0) | - | - |
| Death | 0/19 (0) | 1/33 (3.0) | - | - |
Table 5.
| Variable | Phase I (2010–2014) (n=108) | Phase II (2016–2022) (n=100) | aOR (95% CI)b) | P value |
|---|---|---|---|---|
| Respiratory outcomes | ||||
| Jensen grade | ||||
| Grade 0 | 15/80 (18.8) | 27/63 (42.9) | 3.46 (1.53–7.83) | 0.003 |
| Grade 1 | 30/80 (37.5) | 17/63 (27.0) | 0.62 (0.28–1.37) | 0.240 |
| Grade 2 | 27/80 (33.8) | 17/63 (27.0) | 0.76 (0.35–1.62) | 0.470 |
| Grade 3 | 8/80 (10.0) | 2/63 (3.2) | 0.20 (0.03–1.18) | 0.075 |
| Respiratory support at discharge | ||||
| Nasal prong | 33/80 (41.3) | 18/63 (28.6) | 0.61 (0.28–1.29) | 0.193 |
| High flow nasal cannula | 0/80 (0) | 1/63 (1.6) | - | - |
| Tracheostomy | 0/80 (0) | 1/63 (1.6) | - | - |
| Readmission within 12 months due to respiratory causes | 25/80 (31.3) | 19/63 (30.2) | 1.10 (0.51–2.37) | 0.808 |
| Neurodevelopmental outcomes | ||||
| Death or any NDIa) | 20/88 (22.7) | 15/67 (22.4) | 0.95 (0.42–2.14) | 0.900 |
| Any NDIa) | 13/79 (16.5) | 11/63 (17.5) | 1.17 (0.46–3.02) | 0.740 |
| Cerebral palsy | 11/79 (13.9) | 9/63 (14.3) | 1.04 (0.38–2.91) | 0.934 |
| Hearing loss | 3/79 (3.8) | 1/63 (1.6) | 0.80 (0.07–9.26) | 0.859 |
| Visual impairment | 0/79 (0) | 1/63 (1.6) | - | - |
| Death | 9/88 (10.2) | 4/67 (6.0) | 0.48 (0.12–1.84) | 0.283 |






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


