Article Contents
| Clin Exp Pediatr > Volume 67(1); 2024 |
|
Abstract
Fig. 2.
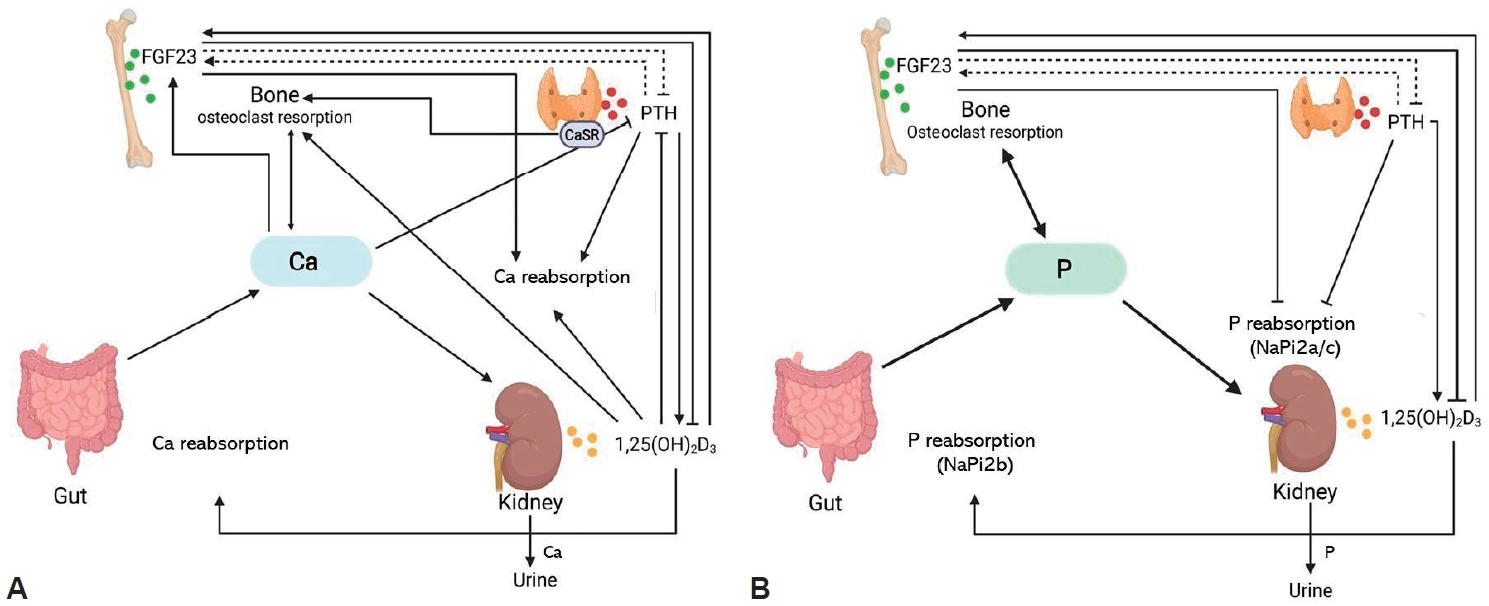
Fig. 3.
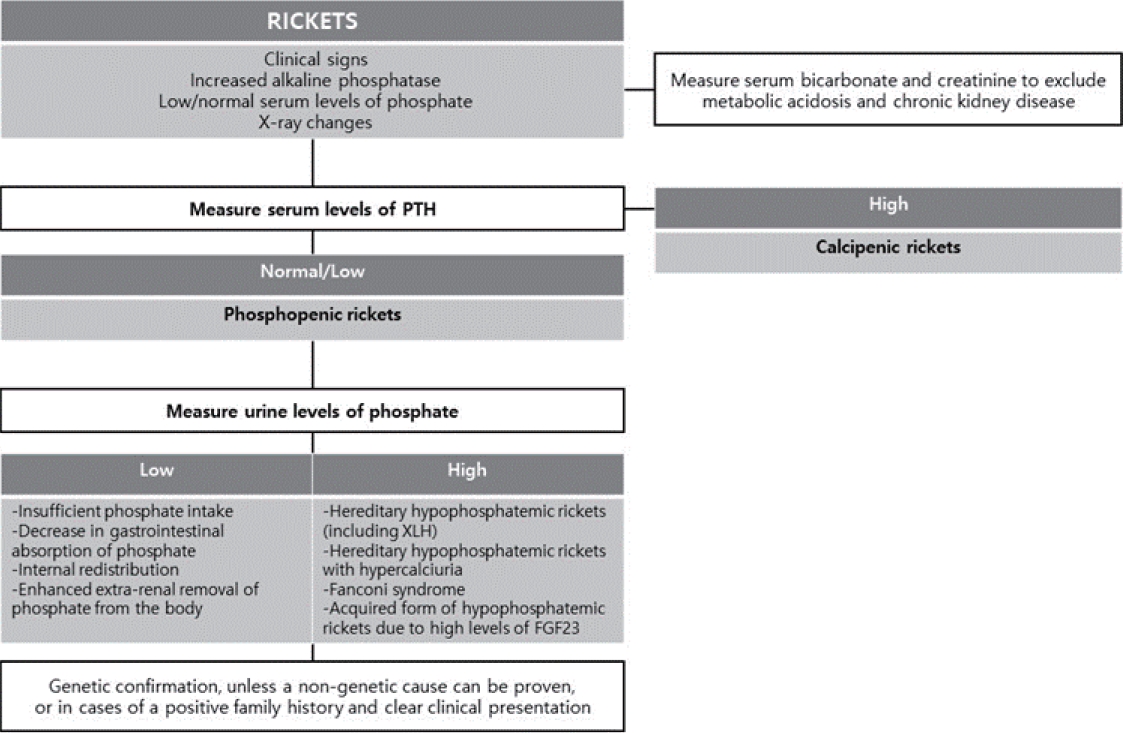
Fig. 4.
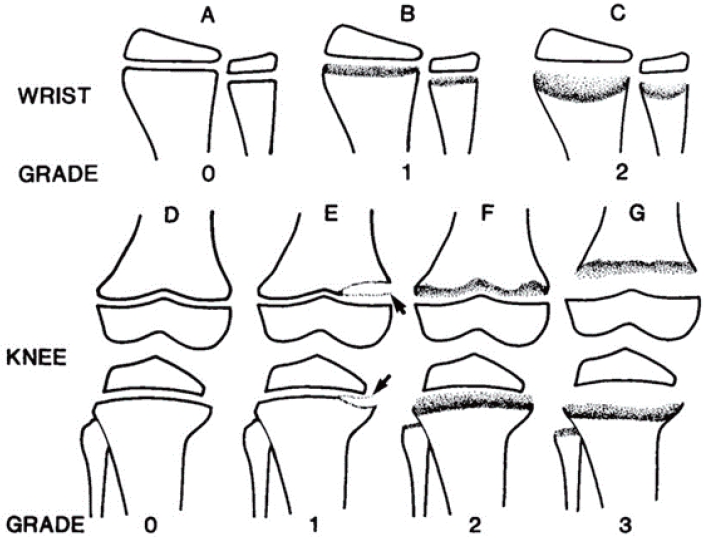
Table 1.
| Disorder (abbreviation; OMIM#) | Gene (location) | Ca | P | ALP | UCa/Crea | UP/Crea | TmP/GFR | FGF23 | PTH | 25(OH)Da) | 1,25(OH)2D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| X-linked hypophosphatemia (XLH; OMIM#307800) | PHEX (Xp22.1) | N | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | ↑, N | N,↑c) | N | Nb) |
| AD hypophosphatemic rickets (ADHR; OMIM#193100) | FGF23 (12p13.3) | N | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | ↑, N | N,↑c) | N | Nb) |
| AR hypophosphatemic rickets 1 (ADHR1; OMIM#241520) | DMP1 (4q22.1) | N | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | ↑, N | N,↑c) | N | Nb) |
| AR hypophosphatemic rickets 2 (ADHR2; OMIM#613312) | ENPP1 (6q23.2) | N | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | ↑, N | N,↑c) | N | Nb) |
| Raine syndrome-associated (ARHR3; OMIM#259775) | FAM20C (7q22.3) | N | ↓ | ↑, ↑↑ | ? | ↑ | ↓ | ↑, N | N,↑c) | N | Nb) |
| Fibrous dysplasia (FD; OMIM#174800) | GNAS (20q13.3) | N, ↓ | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑c) | N | Nb) |
| Tumor-induced osteomalacia (TIO) | NA | N, ↓ | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑c) | N | Nb) |
| Cutaneous skeletal hypophosphatemia syndrome (SFM; OMIM#163200) | NRAS (1p13.2) | N, ↓ | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑c) | N | Nb) |
| HRAS (11p15.5) | |||||||||||
| KRAS (12p12.1) | |||||||||||
| Osteoglophonic dysplasia (OGD; OMIM#612089) | FGFR1 (8p11.23) | N | ↓ | ↑, N | N | ↑ | ↓ | N | N,↑c) | N | Nb) |
| Hypophosphatemic rickets and hyperparathyroidism (OMIM#612089) | KLOTHO (13q13.1) | N | ↓ | ↑, ↑↑ | ↓ | ↑ | ↓ | ↑ | ↑↑ | N | Nb) |
| Rickets and/or osteomalacia with high PTH levels (calcipenic rickets) | |||||||||||
| Nutritional rickets or Vitamin D-dependent rickets | N, ↓ | N, ↓ | ↑, ↑↑ | ↓ | Varies | ↓ | N,↓ | ↑↑↑ | ↓↓, N | Varies | |
Ca, calcium; P, phosphorous; ALP, alkaline phosphatase; UCa/Crea, urinary calcium to creatinine ratio; UP/Crea, urinary phosphate to creatinine ratio; TmP/GFR, maximum rate of renal tubular reabsorption of phosphate normalized to the glomerular filtration rate; FGF23, fibroblast growth factor-23; NA, not applicable; PTH, parathyroid hormone; 25(OH)D, calcidiol; 1,25(OH)2D, 1,25-dihydroxyvitamin; N, normal; ↑, elevated; ↑↑, very elevated; ↑ (↑↑), may range widely.
c) PTH may be moderately elevated; Modified from Haffner D, et al. Pediatr Nephrol 2022;37:2013-36. [2]
Table 2.
| Drug | Dosage |
|---|---|
| Phosphatea) given in 4–6 doses | Elemental P 20–60 mg/kg/day (0.7–2.0 mmol/kg/day) |
| Maximum 80 mg/kg | |
| Calcitriolb) given in 1–2 doses | 20–30 ng/kg/day, alternatively, 0.5 μgc) (age > 12 months) |
| Alphacalcidiolb) (ng/kg) given once daily | 30–50 ng/kg/day, alternatively, 1 μgc) (age > 12 months) |
| Vitamn D2 or D3 | In case of vitamin D deficiency |
| Age-appropriate daily calcium intake | 500-mg Ca in children >12 months |
a) Based on elemental phosphorus; infants and young children usually require more frequent phosphate administrations than older children and adolescents.
b) Phosphate should always be given in combination with either calcitriol or alphacalcidiol or alphacalcidiol.
c) Starting dose; other forms of fibroblast-growth factor 23-associated hypophosphatemic rickets are usually treated with similar doses, but evidence-based recommendations or consensus statements are lacking.
Modified from Haffner et al. Pediatr Nephrol 2022;37:2289-302. [31]
Table 3.
| Examination | 0–5 Years | 5 Years-start of puberty (9–12 years) | Puberty |
|---|---|---|---|
| Frequency of visits | Monthly-3 monthly | 3–6 Months | 3 Months |
| Height, weight, IMD, ICDa) | V | V | V |
| Head circumference and skull shape | V | NA | NA |
| Presence of rickets, pain, stiffness, fatigue, muscle weakness, gait pattern | V | V | V |
| Neurological examinationb) | V | V | V |
| Orthopedic examination | Once a year in the presence of significant leg bowing | ||
| Dental examination | Twice-yearly after tooth eruption | Twice-yearly | Twice-yearly |
| Hearing test | Not feasible | From 8 years: hearing evaluation if symptoms of hearing difficulties | |
| Serum levels of ALP, Ca, Pi, PTH, Crea; eGFR | V | V | V |
| 25(OH) vitamin D levels | After 3–4 weeks in nutritional rickets, yearly in other rickets forms | ||
| 1,25(OH)2 vitamin D levels | Every 3–6 months in patients on burosumab treatment, those on active vitamin D | ||
| UCa/Crea TmP/GFR | Every 3–6 months in patients on active vitamin D or burosumab treatment. Initially, at every visit in patients on burosumab treatment | ||
| Blood pressure | Twice yearly | ||
| Kidney ultrasonography | Every 1–2 years on phosphate, active vitamin D or burosumab treatment | ||
| Left wrist and/or lower limbs radiographs | If leg bowing does not improve upon treatment | In adolescents with persistent lower limb deformities when they are transitioning to adult care | |
| If surgery is indicated | |||
| Dental orthopantomogram | Not feasible | Based on clinical needs | |
| Funduscopy and brain MRI | If aberrant shape of skull, headaches, or neurological symptoms | If recurrent headaches, declining school/cognitive performances, or neurological symptoms | |
IMD, intermalleolar distance; ICD, intercondylar distance; NA, not applicable; ALP, alkaline phosphatase; Ca, calcium; Pi, inorganic phosphate; PTH, parathyroid hormone; Crea, creatinine; eGFR, estimated glomerular filtration rate; 25(OH)D, calcidiol; 1,25(OH)2D, 1,25-dihydroxyvitamin; UCa/Crea, urinary calcium to creatinine ratio; TmP/GFR, maximum rate of tubular reabsorption of phosphate per glomerular filtration rate; MRI, magnetic resonance imaging.
TmP/GFR is calculated by entering the fasting urine and plasma concentrations, in the same concentration units, into the following equation: TmP/GFR=Pp–(Up/Ucr)×Pcr. TmP/GFR, ratio of tubular maximum reabsorption of phosphorus to glomerular filtration rate; Pp, plasma phosphate; Up, urine phosphate; UCr, urine creatinine; PCr, plasma creatinine
b) Consequences of craniosynostosis and spinal stenosis. Modified from Haffner et al. Pediatr Nephrol 2022;37:2289-302. [31]
Table 4.
Total possible score is 10, with 4 for the wrist, and 6 for the knee.
Adapted from Thacher et al. J Trop Pediatr 2000;46:132-9, [53] with permission from Oxford University Press.





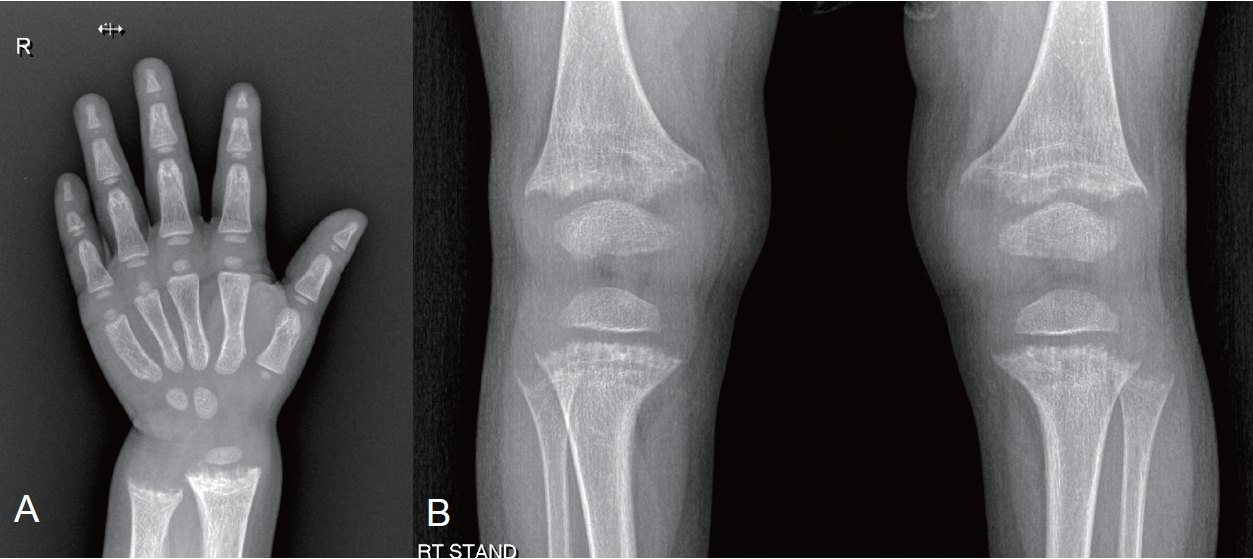
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


