Article Contents
| Clin Exp Pediatr > Volume 66(7); 2023 |
|
Abstract
Background
Chronic constipation is common among children worldwide. Constipation includes functional constipation (FC) and organic constipation (OC). The early recognition of the causes of childhood constipation and its subsequent complications is important.
Purpose
This study aimed to evaluate the prevalence and causes of childhood constipation and compare the clinical characteristics, treatment, and outcomes of children with FC versus OC to identify the predictive factors.
Methods
This retrospective cross-sectional study analyzed children with FC or OC diagnosed in pediatric gastroenterology clinics, Salmaniya Medical Complex, Bahrain, 2017ŌĆō2021. The Rome IV criteria were used to define FC.
Results
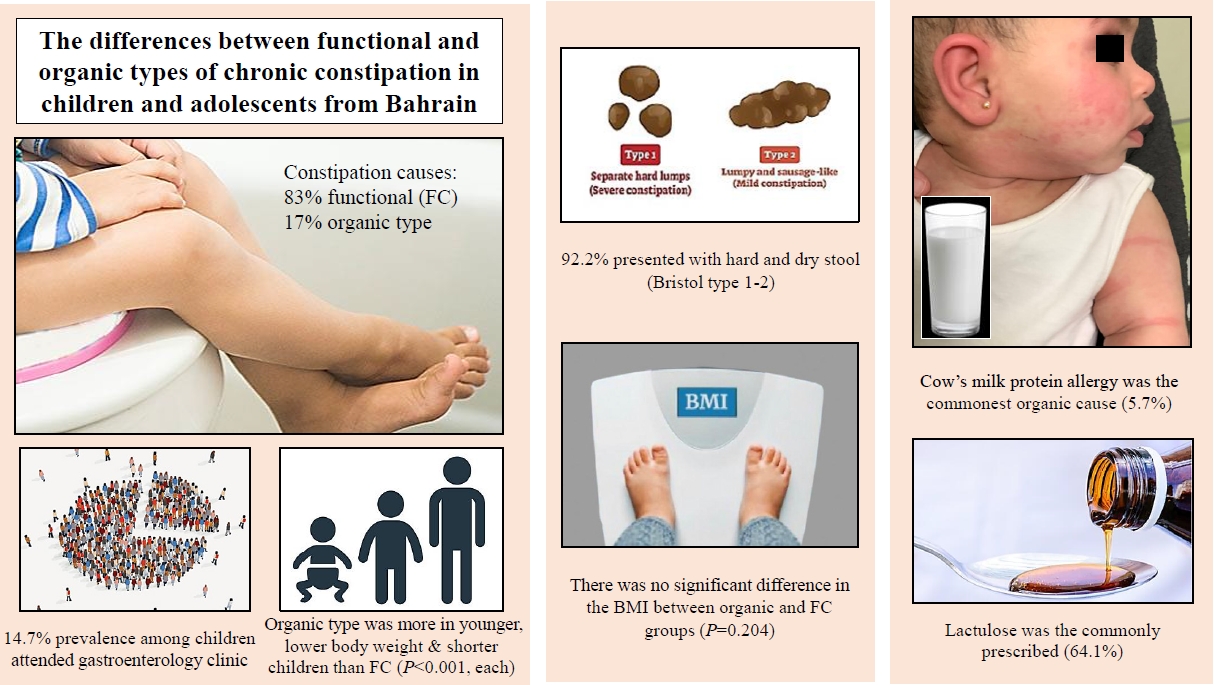
A total of 7,287 gastroenterology appointments were attended by 4,346 children during the study period. Of the 639 children (14.7%) with constipation, 616 (96.4%) were included in the study. Most patients had FC (n=511, 83%), whereas 17% (n=105) had OC. FC was more common in females than in males. Children with OC were younger (P<0.001) and had lower body weights (P<0.001), more stunted growth (P<0.001), and more associated diseases (P= 0.037) than those with FC. Enuresis was the most associated disease (n=21, 3.4%). Organic causes included neurological, allergic, endocrine, gastrointestinal, and genetic diseases. Allergies to cow milk protein were the most common (n=35, 5.7%). The presence of mucus in the stool was more common in OC than in FC (P=0.041), but no other symptoms or physical findings differed. A total of 587 patients (95.3%) received medication, among which lactulose was commonly prescribed (n=395, 64.1%). There were no intergroup differences in nationality, sex, body mass index, seasonal variation, laxative type, or treatment response. A good response was observed in 114 patients (90.5%).
Graphical abstract. Differences between functional and organic chronic constipation in children and adolescents from Bahrain. BMI, body mass index.
Regular defecation is one of the markers of good health [1]. Defecation pattern might vary according to age and dietary habits [1,2]. Hence, it is very important to understand age-related normal and abnormal defecation patterns [1]. Constipation in children is a prevalent and growing problem worldwide [1-6]. Constipation is a commonly overlooked health problem [7]. However, it is one of the most common reasons to visit pediatric clinics and it places a significant burden on primary and secondary healthcare [8-10]. Moreover, it causes anxiety for both children and parents as it affects the psychological, physical, and social wellbeing of the child, worsens academic performance and deteriorates the quality of life [3,11,12]. To eliminate parental anxiety, knowledge about normal defecation patterns in infants and children is important to discriminate the normal from abnormal [1].
Chronic constipation is divided into functional constipation (FC) and organic constipation (OC) [13]. Only a small minority of children has an OC which is diagnosed during the neonatal period [3,14]. FC is the most common beyond the neonatal period [3,4]. Yet, the pathogenesis of FC remains unclear [15]. The most frequent cause of FC appears to be an acquired behavior of withholding of stools and adopting retentive posturing after the experience of painful defecation [3,15]. This leads to fecal stasis with reabsorption of fluid in the colon, causing the stool to become bulky, firm, and painful to pass [3]. Subsequently, stretching of the rectal wall to accommodate the retained feces can lead to decrease in the rectal sensation, and fecal incontinence may develop [3]. Constipation becomes chronic through a vicious cycle of stool retention, painful defecation, stool avoidance and hardening of retained stool through fluid reabsorption [16]. Therefore, early detection of constipation, its cause, and its subsequent complications in children is of great importance [17].
The prevalence of childhood constipation in developing countries is difficult to be estimated as it is dependent on the criteria used to define constipation [12,17,18]. Moreover, childhood FC is perceived as unimportant, easily treatable disorder that improves by its own or needs non-invasive therapy [12]. Furthermore, only a small proportion of patients seek medical advice [12]. Accordingly, the aim of this study was to evaluate the prevalence and causes of childhood chronic constipation, and to compare children and adolescents referred to pediatric gastroenterology clinics for FC or OC in terms of clinical presentations, treatment modalities, and outcomes, to identify the clinical predictors of each type.
A retrospective cross-sectional analytical review of electronic medical records of children diagnosed with chronic constipation in 2 pediatric outpatient gastroenterology clinics, Salmaniya Medical Complex (SMC), Bahrain, between September 2017 and December 2021 was conducted.
All children who fit the definition of chronic constipation at the time of diagnosis were included in the study. Rome IV criteria were used for the diagnosis of functional type of constipation. Accordingly, patients must have experienced at least 2 or more of the following: straining, lumpy or hard stools (Bristol Stool Form Scale 1ŌĆō2), sensation of incomplete evacuation, sensation of anorectal obstruction, the use of manual maneuvers to facilitate defecation (e.g., digital evacuation, and/or support of the pelvic floor) in more than 25% of defecations each; fewer than 3 spontaneous bowel movements per week, loose stools are rarely present without the use of laxatives, and insufficient criteria for irritable bowel syndrome. Moreover, these symptoms could not be fully explained by another medical condition after an appropriate evaluation. These criteria must be fulfilled for the last 3 months, with symptoms onset at least 6 months prior to diagnosis [19]. Patients found to have a medical condition known to cause constipation were considered to have an OC. Patients with no detailed relevant clinical data were excluded.
Demographic data about sex, nationality, age at the time of study, age at diagnosis, date at diagnosis, duration of symptoms, anthropometric measurement including (patientŌĆÖs weight, height, and body mass index [BMI]) and the number of clinic visits were collected. The BMI was calculated as weight in kilograms divided by height in meters squared. The ŌĆ£WHO AnthroPlusŌĆØ anthropometric software program version 3.2.2 (World Health Organization [WHO], Geneva, Switzerland, 2011) was used to calculate the patientŌĆÖs growth parameters. Weight for age z score; height for age z score, and BMI z score were calculated. The growth parameters were presented as a standard deviation (SD) from age- and sex-specific reference means. WHO child growth standards from birth-5 years of age, and WHO growth references for school-age children and adolescents from 5ŌĆō19 years of age were used as references for interpretation of the nutritional status [20,21]. Accordingly, thinness were defined as BMI <-2 SD while possible risk of overweight, overweight and obesity were defined as BMI for age >+1, >+2 and >+3 SD, respectively. Linear growth impairment (stunting) was defined as length/height for age <-2 SD.
Date at presentation was used to study the effect of climate on the onset of constipation. According to Bahrain authority for culture and antiquities, the climate in Bahrain has 2 seasons, an extremely hot summer (May to October) where the temperature ranges between 37┬░C and 40┬░C or a relatively mild winter (November to April) with a temperature range between 10┬░C to 25┬░C.
PatientŌĆÖs present history including frequency of bowel movement, stool consistency, presence of blood or mucus in the stool, straining, painful defecation, rectal prolapse, anal itching, soiling, history of abdominal pain, colic, loss of appetite, nausea, vomiting, urinary tract infections (UTIs), urinary incontinence, family history of chronic constipation, history of consumption of iron therapy, high-fiber diet and milk consumption were obtained.
History of associated chronic diseases such as HirschsprungŌĆÖs disease, gastroesophageal reflux disease (GERD), gastritis, hypothyroidism, trisomy 21, cowŌĆÖs milk protein allergy (CMPA), atopy, attention deficit hyperactivity disorders, autism, cerebral palsy, hydrocephalus, meningomyelocele, epilepsy, sickle cell disease (SCD), glucose-6-phosphate dehydrogenase deficiency and thalassemia were gathered.
Physical examination findings such as abdominal distention, rectal mass, perianal fissures, perianal skin tags, sacral dimples or depressions, and hair tufts were included. Physical deformities such as rectal prolapse, umbilical hernia, hypotonia, anal stenosis, and lumbosacral scars of previous spinal surgeries were noted.
Organic causes of childhood constipation were categorized according to the MSD Manual Professional Version website [22]. Accordingly, organic causes of constipation in infants and children were classified into anatomic causes, endocrine or metabolic disorders, spinal cord defects, intestinal disorders, severe neurologic deficits, drug adverse effects, and toxins induced constipation. Medical therapies including previous use of laxatives, use of disimpactive drugs, and maintenance therapy were documented. PatientŌĆÖs adherence to medical therapies (strict or poor), response to medications and follow-up duration were gathered. The response to treatment was classified into good or poor response according to the improvement of symptoms after using one or more type of laxatives for treatment of constipation.
PatientsŌĆÖ data were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). PatientŌĆÖs age was classified into 3 groups: preschool (below 5 years), school (5 to 10 years), and adolescents (above 10 years). Frequency and percentages were calculated for categorical variables. Continuous variables were presented as mean┬▒SD or median and interquartile range (IQR) according to distributionŌĆÖs normality. Patients were divided into 2 groups according to the type of constipation (FC or OC) and both groups were compared constipation to their demographic data, anthropometric growth parameters, weather season at presentation (summer or winter), clinical presentations, physical findings, and the type of laxative used. Fisher's exact tests or Pearson's chi-square were used to compare categorical variables while Mann-Whitney U test was used to compare continuous variables. P values <0.05 were considered statistically significant.
During the study period, 7,287 appointments were booked in pediatric gastroenterology outpatient clinic which were attended by 4,346 patients. Of the latter, 639 patients (14.7%) attended the clinics because of chronic constipation. Twentythree patients (3.6%) were excluded; 20 of them had duplicate records, 2 patients did not fulfill the inclusion criteria and they were below the age of 1 month, and 1 patient was excluded due to missing all data. The remaining 616 patients (96.4%) were included in the study. Based on 2020 Bahrain health statistics, of 1,472,204 total population of Bahrain, 481,819 were children aged below 18 years. Accordingly, the overall prevalence of chronic constipation in children referred to the tertiary gastrointestinal clinics was 0.13% (127.9 patients per 100,000 populations at risk).
Most of the patients had FC (n=511, 83%), while the rest had OC (n=105, 17%). Demographic data of the included patients are shown in Table 1. The majority were Bahraini (n=588, 95.5%]), while the others were non-Bahraini (n=28, 4.5%) (9 patients were from Egypt, 6 from Pakistan, 5 from Syria, 2 from Saudi Arabia, India, and Yemen each, and one was from Jordan and Philippine each). FC was found to be more among females and Bahraini. However, no significant difference was noted between the 2 types of constipation in terms of sex or nationality. The median age at the time of diagnosis was 5.9 years (IQR, 2.3ŌĆō9.2 years) while the median age at the time of study was 10 years (IQR, 6ŌĆō13 years). The most common age group at presentation was below 5 years (n=275, 44.6%). There was a significant difference between FC and OC according to the age group (P<0.001). OC was found to be more in younger children compared to FC with a median age at diagnosis of 3.1 years (IQR, 1.5ŌĆō7.3 years) versus 6.3 years (IQR, 2.7ŌĆō9.4 years), respectively, P<0.001. There was no difference in BMI between the OC and the FC groups (P=0.204) or the weather seasons (P=0.592) at the onset of constipation. Yet, patients with OC were significantly lower in body weight (P<0.001) and more stunted (P<0.001) than those with FC. The analysis of growth parameters is shown in Table 1. There was no significant difference between FC and OC in terms of obesity. However, when we combined patients with possible risk of overweight, overweight and obesity and compare them with thinness and normal weight patients, children with FC were more in the higher weight group (P=0.023).
The organic causes of constipation are shown in Table 2. The overall organic causes were in order of neurological causes, allergies, endocrine, gastrointestinal, and genetic diseases. However, CMPA was specifically the commonest (n=35, 5.7%), followed by cerebral palsy (n=27, 4.4%) and hypothyroidism (n=15, 2.4%]). Some patients had more than one organic cause.
One hundred fifty-five patients (25.2%) had one or more associated diseases that were not considered as a cause for constipation. Children with OC had higher percentage of associated diseases (n=120, 33.3%) than those with FC (n=35, 23.5%) which was statistically significant (P=0.037). The most common associated disease was enuresis (n=21, 3.4%), followed by SCD (n=19, 3%), and GERD (n=18, 2.9%). Other associated diseases are shown in Supplementary Table 1.
Comparison of patientŌĆÖs history and physical examination between functional and OC groups is shown in Table 3. Relevant history data was available in 589 patients (95.6%). The most common presenting symptom in both types of constipation was passage of hard and dry stool; followed by recurrent abdominal pain in FC or vomiting in OC. The presence of ŌĆ£mucus with stoolŌĆØ was more in OC (P=0.041). Yet, no significant differences were found in the other presenting symptoms. In terms of dietary pattern, 99 (16%) out 142 patients (23%) with available data were consuming low fiber diet (89 of 124 patients [71.8%] in FC group versus 10 of 18 [55.6%] in the OC group, P=0.177). Data about milk consumption was available in 52 patients (8.4%), 14 (26.9%) of them had a history of high consumption (11 of 35 patients [34.4%] in FC group versus 3 of 17 [17.7%] in the OC group, P=0.341). Data about family history was positive for constipation in one patient with FC out of 17 with available data (5.8%). Physical examination was unremarkable in most of the patients (n=494, 80.2%) while positive physical findings were detected in 93 patients (15.1%), 80 (19.5%) from the FC group and 13 (12.4%) from the OC group. The most frequent finding in both groups was abdominal distension (n=56, 9.1%) followed by perianal fissure (n=33, 5.4%). In terms of the deformities, rectal prolapse (n=5) and umbilical hernia (n=1) were found only in patients with FC while hypotonia (n=3), anal stenosis (n=3), and meningomyelocele scar (n=2) were found only in those with OC.
Of 616 patients, 587 (95.3%) received medications for treatment of constipation along with dietary advice. Some patients required more than one laxative for treatment of constipation. Prior to the outpatient visits, 136 patients (22%) had a history of laxative use either from primary healthcare clinic or from the Emergency Department. The most common laxative used prior to the visit was lactulose (n=87, 64%), followed by magnesium hydroxide (n=22, 16.2%), polyethylene glycol (n=14, 10.3%), bisacodyl (n=9, 6.6%), glycerin suppositories (n=2, 1.5%), disodium phosphate and herbal medication (n=1, 0.7% each). While 508 patients (82.5%) received treatment after gastroenterology clinic visit. Different types of medical therapy provided by gastroenterologist for treatment of constipation are shown in Table 4. The most common medication used was lactulose (n=395, 64.1%), followed by magnesium hydroxide (n=265, 43%) and glycerin suppository (n=255, 41.4%). Two patients (0.3%) were on iron supplements. No significant differences in the type of laxative used were found between the FC and OC.
Data about response to treatment was available for 126 patients (20.5%), 114 (90.5%) of them had good response while 12 patients (9.5%) had poor response. No significant difference in the good response to laxative use was found between the FC and OC (104 of 115 patients [90.4%] in FC group vs. 10 of 11 [90.9%] in the OC group, P=1.000). Seven patients (5.6%) had poor compliance to medications. Out of 616 patients, 527 (85.6%) required more than 1 follow-up visit, while the remaining 89 (14.4%) attended the clinic once. No difference in the median number of outpatient clinic visits found between the 2 groups (P=0.310). Children with OC required longer follow-up duration compared to those with FC, but this finding was not statistically significant (P=0.059) (Table 1).
The prevalence of chronic constipation among children differs widely in the literature [1]. In this study, the overall prevalence of childhood constipation required a tertiary care in Bahrain was 0.13%. However, it was accounted for 14.7% from the pediatric gastroenterology outpatient visits. This figure is comparable to the study of Madhu et al. [12] where the prevalence was 14.29%. However, Talachian et al. [17], Altamimi [9], Ip et al. [7], Kondapalli and Gullapalli [5], Ma et al. [23], and Haghighat et al. [24] reported a higher prevalence of 15.64%, 25.9%, 29.6%, 30.88%, 60%, and 40.4%, respectively. On the other hand, Kocaay et al. [1] and Park et al. [25] reported a lower prevalence of constipation, 4.7% and 8.5%, respectively. This variation could be attributed to the differences in the setting and the age of patients included in each study.
There are many definitions for chronic constipation. However, no global consensus had been established regarding this issue [6]. As an example, The American College of Gastroenterology defined constipation based upon symptoms that include unsatisfactory defecation with either infrequent stools, difficulty in passing stool, or both [26]. Yet, The Canadian consensus group also defined chronic constipation as being symptom based but with more details, including fewer than 3 stool per week, stool form that is mostly hard or lumpy, and difficult stool passage (that need to strain or incomplete evacuation) for more than 6 months [27]. Nowadays, Rome IV criteria are the most recent criteria used for establish the diagnosis, which was used in this study. However, several previous studies used Rome II or Rome III criteria to define chronic constipation [2,3,5,7-9,11,12,15,23-25,28]. This variation in the definition makes the comparison between the findings of different studies hard to achieve.
Constipation in children could be a result of either functional or multiple organic causes. However, their frequency is not well known [17]. In the present study, most of the patients (n=511, 83%) had the FC. This was also documented in several other studies. However, higher percentage of FC was reported by Talachian et al. (87%) [17], Ali et al. (88.7%) [13], Kocaay et al. (95.8%) [1], and Haghighat et al. (98.7%) [24]. This variation in the percentage can be explained by the study setting. Our study was based on a tertiary setting where more difficult cases are seen, and chance of organic causes might be more.
The current study showed no significant difference between the FC and OC in terms of sex. Yet, females had more FC than males. This is comparable to several other studies. For instance, Haghighat et al. [24], Turco et al. [15], Dehghani et al. [8], and Khalil [4] studies also showed female predominance, with a percentage of 50.2%, 53%, 55.9%, and 56.7% respectively. The reasons for female predominance might be explained by that most girls get embarrassed to use public bathrooms and they withhold stools until they get home. However, several other studies reported higher prevalence in males [1,3,9,11,13,16].
In this study, the median age at the time of diagnosis was 5.9 years (IQR, 2.3ŌĆō9.2 years) with preschool children below 5 years being the most frequent (n=275, 44.6%). Comparably, Kondapalli and Gullapalli [5] and Dehghani et al. [8] reported a mean age at diagnosis of 5.52┬▒3.085 and 5┬▒3.12 years, respectively. However, Haghighat et al. [24], Ip et al. [7], and Park et al. [25] reported constipation in younger children (1.8┬▒2.1, 4.12┬▒0.89, and 4.5┬▒1.25 years, respectively). On the other hand, Fujitani et al. [2], Appak et al. [11] and Sinha et al. [28] reported it in older age (6.5┬▒1.3, 8.6┬▒2.9, and 8.8┬▒4.2 years, respectively). Moreover, like our study, Ali et al. [13], Kondapalli and Gullapalli [5], Bansal et al. [3], and Altamimi [9] studies revealed a higher prevalence of constipation among preschool children with a percentage of 64%, 57.42%, 46.15%, and 43.7%, respectively. Toilet training and the intake of low fiber diet could be the reasons behind this high prevalence in the preschool children [1]. Furthermore, in this study, OC was significantly higher among children below 5 years of age compared to older age in FC group. Moreover, Biggs and Dery [14] reported OC among neonates. This is also supported by Bansal et al. [3] who stated that FC occurs after the neonatal period.
In the current study, there was no significant difference in BMI between FC and OC group. Fujitani et al. [2] and Park et al. [25] reported a mean BMI of 15.7┬▒1.9 and 15.9┬▒2.2 kg/m2, respectively; in children with FC which was also comparable to that of our study (16.1; IQR, 14.7ŌĆō19.1). However, most of the reviewed studies did not compare BMI in children with FC and that of the OC. Moreover, patients with OC in our study had significantly lower body weight and were more stunted than those with FC. This lower weight and height might be explained by the anorexia, malabsorption, and inadequate intake, malpractices of feeding and weaning associated with chronic constipation [12]. On the other hand, as shown in our study, children with FC are more likely to be overweight as they have increased incidence of psychological/behavioral problems [12]. The median weight in patients with FC in this study was 20.8 kg (IQR, 13.3ŌĆō33.5 kg) and the median height was 119 cm (IQR, 93ŌĆō136 cm). These figures were comparable to those reported by Fujitani et al. [2] where the mean weight was 22.2┬▒4.9 kg and the mean height was 118.6┬▒10.0 cm in patients with FC. However, Park et al. [25] reported a lower weight (17.0┬▒5.1 kg) and height (104.3┬▒11.5 cm) in their younger patients with FC.
There are many risk factors for FC such as: not being breastfed, high consumption of cowŌĆÖs milk, low fiber in diet, toilet training before 2 years of age, avoiding school toilets, physical inactivity and positive family history for constipation [1]. In the study of Kocaay et al. [1], breastfed infants found to have significantly lower percentage of constipation. In contrary, Kocaay et al. [1] and Kondapalli and Gullapalli [5] showed that high cowŌĆÖs milk consumption is associated with constipation in children. In the current study, data about milk consumption was available in 52 patients (8.4%), 14 (26.9%) of them had history of high consumption. On the other hand, low fiber diet is also a risk factor. In the present study, 99 patients (16%) were consuming low fiber diet. This factor was also reported in several previous studies [1,2,5,7,12,24,25]. In our study, high milk consumption and low fiber diet were reported in both types of constipation, FC and OC, with no significant difference. Another factor for FC is avoidance of school toilets. Appak et al. [11] revealed that 29.6% of the patients attending school were not using school toilet that led to fecal stasis and the stool become bulky, firm, and painful to pass. Moreover, positive family history for chronic constipation was also an important risk factor. In this study, positive family history was noted in 5.8%. Ip et al. [7], Appak et al. [11], and Kocaay et al. [1] reported a higher percentage of constipation in children with positive family history, 14%, 53.1%, and 54.2%, respectively.
In this study, 105 patients (17%) had an OC. However, Talachian et al. [17], Kocaay et al. [1], and Haghighat et al. [24] reported lower percentage of OC (13%, 4.2% and 1.3%, respectively). This might be also related to the variation in the study setting and patientsŌĆÖ demography among different studies. Studies based on primary care setting will give lower percentage of OC compared to those from secondary or tertiary settings. Moreover, studies of younger age might have higher percentage of organic diseases as shown in our study where younger patients had more organic causes. There are many causes for OC that must be ruled out during the neonatal period [1]. For example: CMPA, cerebral palsy, Hirschsprung disease, anal stenosis, and hypothyroidism. In this study, the most common organic cause was CMPA (5.7%). Similarly, Altamimi [9] reported CMPA as one of the leading causes for OC. CMPA is the most common cause of constipation in the first 3 years of life [29]. The presence of allergic inflammation of the rectal mucosa can lead to increased resting anal sphincter pressure and an abnormal relaxation of the anal canal causing chronic constipation, that disappear after elimination of cowŌĆÖs milk protein from the diet. [30] Cerebral palsy was the second organic cause in this study and found in (4.4%) of the children. In contrary, Haghighat et al. [24] reported a higher percentage of cerebral palsy and it was the commonest cause (38.4%). On the other hand, Bansal et al. [3] reported a lower percentage (1.92%). Hypothyroidism was the third cause and found in 2.4% in the current study, which is higher than the percentage reported by Bansal et al. [3] and Ali et al. [13] (1.28% and 1.2, respectively). Nonetheless, Bansal et al. [3] and Ali et al. [13] showed that HirschsprungŌĆÖs disease was the commonest cause with a percentage of 6.41% and 8%, respectively. This is much higher than the percentage of HirschsprungŌĆÖs disease in this study (0.6%). Moreover, Talachian et al. [17], reported that anal stenosis was the commonest cause (6.9%). However, in this study anal stenosis was found in 0.5% of the patients. Summary of previous studies of chronic constipation in children from neighboring countries and worldwide is shown in Supplementary Table 2.
In the current study, both types of constipation have been associated with other diseases that were not considered as a cause of the disease. Moreover, children with OC had higher percentage of associated diseases (33.3%) than those with FC (23.5%) (P=0.037). This point was not discussed in detail by previous studies to compare with. In general, the commonest associated disease was enuresis (n=21, 3.4%). This might be attributed to the rectal disease which may cause urinary symptoms through mechanical compression of the stool mass over the bladder [9]. Furthermore, children with more severe constipation symptoms had more reduced bladder capacity [23]. Constipated children are 1.47 times more likely to have enuresis when compared to healthy children [23]. Moreover, a study by Appak et al. [11] reported that 43.8% of constipated patients had enuresis. On the other hand, Ma et al. [23] determined that 60% of enuretic children had constipation. So, it is necessary to evaluate constipation in each child with enuresis and vice versa [23].
SCD was found in 19 children (3%) with constipation in this study. Similarly, Chumpitazi et al. [31] reported SCD in 11 out of 512 children (2%) with abdominal pain and constipation. This association might be explained by the frequent painful episodes of abdominal vaso-occlusive crises that occur in these patients which prevent them from straining. On the other hand, abdominal pain due to constipation may be misinterpreted as a worsening vaso-occlusive crisis with subsequent higher doses of narcotics which subsequently worsening constipation [32].
GERD was found in 18 patients (2.9%) in this study. The association between GERD and constipation can be explained by the avoidance of specific food and poor fluid intake, as well as it can be a side effect of using proton pump inhibitors or aluminum hydroxide containing antacids [33,34].
Clinical manifestation of chronic constipation varies among different studies [8]. In this study, the most frequent symptom was passage of hard and dry stool (543 of 589, 92.2%). This finding agreed with the previous studies where the percentage was ranging between 85.26% and 93.7% [3,8,9]. Additionally, hard stool consistency is associated with painful defecation [1]. In this study, painful defecation was noted in only 7.6% (45 of 589) of patients. However, painful defecation was more frequent in Fujitani et al. (22.7%) [2], Haghighat et al. (60.75%) [24], Dehghani et al. (92.3%) [8], and Appak et al. (96.9%) [11] studies, respectively.
Recurrent abdominal pain was the second symptom reported in this study (227 of 589, 38.5%). This percentage is comparable to that reported by Kondapalli and Gullapalli [5], Altamimi [9], and Dehghani et al. [8] where recurrent abdominal pain represented 30.6%, 40%, and 41.4% of their population, respectively.
In the current study, 20.8% of the patients presented with straining. However, in the study of Ali et al. [13], straining was noted in 43%. This difference can be attributed to that many cases with a withholding behavior have been misinterpreted by the parents as an attempt of straining for defecation, and that prevent anal relaxation rather than pushing the stool down [9,13]. Withholding behavior was one of the most frequent symptoms reported by Dehghani et al. [8] which was found in 92.3% of their patients.
Soiling is a common symptom of constipation in childhood that cause psychosocial difficulties and stress within families [1,11]. The present study showed that 9.8% (58 of 589) of patients had soiling. However, several studies reported higher percentage of soiling among constipated children ranging between 16.7% and 58.33% [1,3,5,8,11,12,17,24]. This might be related to the severity of constipation [11]. Moreover, soiling might be interpreted by parents or even by physicians as diarrhea which might underestimate this advanced stage of constipation.
In this study, rectal bleeding was found in 8.7% (51 of 589) of patients. Rectal bleeding may be due to perianal fissure or hemorrhoids. In the study of Kondapalli and Gullapalli [5], blood-streaked stools were present in 10.89% of the constipated children which is also comparable to the percentage of our study.
In the current study, UTI was found in 3% of patients. However, Kocaay et al. [1] reported higher prevalence of recurrent UTI in their constipated children (8.3%). This might be related to the elevated rectal fecal load that change the physiological neural stimuli of the bladder leading to chronic bladder spasm, insufficient emptying and significant post-void urine volumes [35]. Moreover, wiping from back to front after a bowel movement cleaning, instead of front to back, might be the reason behind UTI, which is more frequent in girls [36].
This study showed no significant differences in most of the presenting symptoms between FC and OC groups apart from ŌĆ£mucus with stoolŌĆØ which was more in the OC (P=0.041). Presence of ŌĆ£mucus with stoolŌĆØ or in another term ŌĆ£colitisŌĆØ might indicate presence of underlying CMPA. However, this finding should be interpreted with caution due to presence of less than 20 patients in each type of constipation.
In this study, the most frequent physical finding was abdominal distension (n=56, 9.1%) which was noted in both types of constipation. Comparably, the study of Kocaay et al. [1] showed abdominal distension in 6.3% of their patients. However, most of the previous studies reported fecal rectal masses as the main noticeable physical finding [3,8,9,11,24,25]. This might be related to the fact that per rectum examination was not routinely performed in our institution in children with constipation. Nonetheless, the second frequent physical finding in this study was perianal fissure (n=33, 5.4%). This finding is comparable to the study of Dehghani et al. [8] (7.2%). However, Kocaay et al. [1] reported anal fissure in 35.4%. Perianal lesions such as perianal fissure and rectal prolapse can be considered as complications secondary to FC.
Treatment of constipation in children with laxatives involves 3 steps: disimpaction, maintenance and weaning [37]. In this study, 587 patients (95.3%) received medical therapy. The most frequently used medication in both groups was lactulose which was prescribed for 64.1% of the patients. Likewise, Hasosah et al. [10] found that lactulose was the most used laxative. Lactulose is a synthetic disaccharide and it is effective at normalizing the frequency and consistency of the stool [14,27]. Moreover, lactulose is considered safe for all age groups, and it is recommended if polyethylene glycol is not available. Magnesium hydroxide was the second laxative used in the current study (43%). However, it was not the case in the previous studies. This might be related to the possible side effects of this drug and/or its availability in their hospitals. Glycerin suppository was used in 41.4% of the patients in this study being the third prescribed medication. It belongs to a class of hyperosmolar laxatives and can be given if symptoms were not relieved after increasing fiber intake [27]. Glycerin suppositories are given to initiate rectal evacuation and it can be used as long as needed [27]. Despite that glycerin suppository was the second most common medication used in their study; Hasosah et al. [10] reported that pediatricians are using them less frequently compared to other physicians. In the studies of Haghighat et al. [24] and Dehghani et al. [8], polyethylene glycol was the most commonly used medication and was prescribed for 60% and 70.3% of their patients, respectively. However, in this study polyethylene glycol was used only in 6.3% of children. This might be related to the higher price of polyethylene glycol compared to other laxatives. Also, this medication is not routinely available in our governmental hospitals.
In the current study, more than 90% of patients in both groups had good response to treatment. The response to treatment can be affected by multiple factors such as genetic background, dietary habits, compliance to medications and toileting behavior. Genetic variations in the metabolism of drugs might eventually lead to poor response [6]. Moreover, some patients may have late response to medications. In addition, some mothers prefer the natural ways of treatment by increasing fibers in diet or using herbal medications rather than the medications prescribed by their pediatrician. In this study, poor compliance to medication was low as noticed in 7 patients (1.1%) which explained the high rate of good response.
In this study, the median follow-up period of children with constipation was 1.4 years (IQR, 0.64ŌĆō2.7 years) with a median number of visits of 4 (IQR, 2ŌĆō6). Moreover, children with OC required longer follow-up duration compared to those with FC but this finding was not statistically significant. This reflects the long duration and the chronicity of this condition which requires long-term management. This also confirms Bansal et al. [3] finding where the average duration of constipation was 1.64 years. However, other studies reported a longer mean duration of constipation ranging between 2.2┬▒1.9 to 4.3┬▒3.6 years [8,11,24,28]. In terms of number of outpatient visits, Sinha et al. [28] showed that the mean number was 6.6┬▒7.5 visits, even before referral to outpatient gastroenterology clinic.
This study was limited by the lack of a generally accepted definition for chronic constipation among different studies [1]. In this study, the latest Rome IV criteria was used to define FC while most of the other studies used the old criteria (Rome II or III) or even no criteria was mentioned (Supplementary Table 2), which makes our study unique in this regard. Yet, this jeopardized the comparison with other studies. Moreover, risk factors such as timing of first meconium passage, gestational age, psychological problems, and age at toilet training are important risk factors for constipation. However, they were missing in this study. Furthermore, although breast feeding is considered a protective factor against development of constipation, data about breast feeding was also missing in this study. In addition, it is a single tertiary center study, including only patients attended outpatient clinics and patients that have been admitted to the hospital were not included, which are more severe patients. Another limitation, some patients missed their follow-up visits during corona virus pandemic which made calculation of the duration of constipation after the treatment is incomplete. Despite these limitations, this study is the first study in Bahrain about chronic constipation in children, with a relatively large number of patients. Moreover, constipation in children is underreported in the Middle East, which is reflected by the number of published related manuscripts that are scarce; therefore, any publication tackling childhood constipation from the Middle East is of a great value. Furthermore, this study covers both types of chronic constipation (functional and organic) while most of the published studies reported only one of these types, and mainly the functional type [2,4,5,8,11,12,15,23,25,27,28]. In addition, it covers all aspects of chronic constipation starting from clinical presentation until the patientŌĆÖs outcome along with the clinical predictors that can help to differentiate the OC from the FC. Moreover, in this study, different types of laxatives were prescribed as a treatment for chronic constipation, and the commonest was lactulose. On the other hand, most of the previous studies mentioned limited types of laxatives, and polyethylene glycol 3350 was commonest used [4,8,28]. The findings of this study are very important for any primary care doctor, pediatrician, or gastroenterologist as they can direct their attention toward investigating younger children and those with lower anthropometric growth parameters for age, or a positive history of mucus in the stool, which might indicate an underlying organic cause of constipation. It can also help them in developing clinical guidelines and polices regarding the prevention and treatment of a common problem in children. The findings of this study can aid any future systematic review that pools data from different countries and can form a strong foundation of any future research.
In conclusion, chronic constipation is a common problem in children, and it represents a significant portion of outpatient gastroenterology visits. Although, some findings of our study were similar to those of other studies published worldwide such as some demographic characteristics, anthropometric parameters, FC predominance, main symptoms of constipation, and good response to treatment, it was different from other studies in many aspects. Our study used the most recent ROME IV criteria to define FC, covered both types of constipation (FC and OC), the main physical finding was abdominal distension, the commonest organic cause was cowŌĆÖs milk protein allergy, and the most frequent type of laxative used was lactulose. Our study also suggested some clinical predictors that might help to differentiate the OC from the FC. Younger children aged below 5 years, those with low body weight and stunted, those with a history of mucus in stool, and those with associated diseases should be assessed for an underlying organic cause such as neurological causes, allergies, endocrine diseases, or others. Further studies are required to identify other possible risk factors, and to determine the response to each type of medical therapy.
Supplementary material
Supplementary Tables 1 and 2 can be found via https://doi.org/10.3345/cep.2022.01298.
Supplementary┬ĀTable┬Ā1.
Associated diseases in 616 children with chronic constipation
Supplementary┬ĀTable┬Ā2.
Summary of previous studies of chronic constipation in children from neighboring countries and worldwide
Footnotes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: Isa HM, Alkharsi FA; Data curation: Isa HM, Alkharsi FA, Salman FA, Ali MS; Formal analysis: Isa HM, Alkharsi FA; Funding acquisition: Isa HM, Alkharsi FA; Methodology: Isa HM, Alkharsi FA; Project administration: Isa HM, Alkharsi FA, Mohamed AF; Visualization: Isa HM, Alkharsi FA, Mohamed AF; Writing original draft: Isa HM, Alkharsi FA, Salman FA, Ali MS; Writing - review & editing: Isa HM, Alkharsi FA, Salman FA, Ali MS, Abdulnabi ZK, Mohamed AF.
Acknowledgments
The authors gratefully acknowledge all those who provide care for children with chronic constipation in the pediatric department, Salmaniya Medical Complex, Kingdom of Bahrain. The authors also thank staffs in the medical record department for providing the list of outpatient visits.
Table┬Ā1.
Demographic data of children with functional versus organic constipation
| Variable | Total (n=616) | Functional (n=511, 83%) | Organic (n=105, 17%) | P value | |
|---|---|---|---|---|---|
| Nationality | 0.299a) | ||||
| Bahraini | 588 (95.5) | 490 (95.9) | 98 (93.3) | ||
| Non-Bahraini | 28 (4.5) | 21 (4.1) | 7 (6.7) | ||
| Sex | 0.134a) | ||||
| Female | 306 (49.7) | 261 (51.1) | 45 (42.9) | ||
| Male | 310 (50.3) | 250 (48.9) | 60 (57.1) | ||
| Age group (yr) | <0.001b) | ||||
| <5 | 275 (44.6) | 209 (40.9) | 66 (62.9) | ||
| 5ŌĆō10 | 221 (35.9) | 196 (38.4) | 25 (23.8) | ||
| >10 | 120 (19.5) | 106 (20.7) | 14 (13.3) | ||
| Anthropometric measurements | |||||
| Weight (kg) | 528 (85.7) | 20.8 (13.3ŌĆō33.5) | 13.5 (9.8ŌĆō20.3) | <0.001c) | |
| WA z score | 430 (69.8) | 0.3 (-0.7 to 1.7) | 0.4 (-1.4 to 1.3) | 0.160c) | |
| Height (cm) | 491 (79.7) | 119 (93ŌĆō136) | 93.3 (78.3ŌĆō119.5) | <0.001c) | |
| HA z score | 491 (79.7) | 0.4 (-0.7 to 1.6) | 0.3 (-1.8 to 2.0) | 0.341c) | |
| BMI (kg/m2) | 491 (79.7) | 16.1 (14.7ŌĆō19.1) | 16.1 (14.3ŌĆō17.7) | 0.204c) | |
| BMI z score | 491 (79.7) | 0.1 (-0.9 to 1.5) | -0.1 (-1.3 to 9.2) | 0.111c) | |
| WHO growth parameters classification 2006 | 491 (79.7) | 411 (80.4) | 80 (76.2) | ||
| Thinness (BMI z score <-2 SD) | 50 (8.1) | 37 (9.0) | 13 (16.3) | 0.067a) | |
| Normal weight (BMI z score = -2-1 SD) | 297 (48.2) | 245 (59.6) | 52 (65.0) | 0.385a) | |
| Risk of overweight (BMI z score > +1 SD) | 63 (10.2) | 58 (14.1) | 5 (6.3) | 0.066a) | |
| Overweight (BMI z score > +2 SD) | 38 (6.2) | 31 (7.5) | 7 (8.8) | 0.652a) | |
| Obesity (BMI z score > +3 SD) | 43 (7.0) | 40 (9.7) | 3 (3.8) | 0.088a) | |
| Normal height (HA z score Ōēź -2 SD) | 442 (71.8) | 380 (92.5) | 62 (77.5) | <0.001a) | |
| Stunting (HA z score < -2 SD) | 49 (8.0) | 31 (7.5) | 18 (22.5) | <0.001a) | |
| Season at presentation | 0.592b) | ||||
| Summer (May to October) | 292 (47.4) | 245 (47.9) | 47 (44.8) | ||
| Winter (November to April) | 324 (52.6) | 266 (52.1) | 58 (55.2) | ||
| Associated diseases | 155 (25.2) | 120 (23.5) | 35 (33.3) | 0.037a) | |
| No. of outpatient visits | 616 (100) | 4 (2ŌĆō6) | 4 (2ŌĆō7) | 0.310c) | |
| Follow-up duration (yr) | 616 (100) | 1.3 (0.6ŌĆō2.5) | 1.7 (0.8ŌĆō3.3) | 0.059c) | |
Table┬Ā2.
Causes of organic constipation (n=616 children)
| Cause | No. (%) |
|---|---|
| Neurological diseases | 39 (6.3) |
| ŌĆāCerebral palsy | 27 (4.4) |
| ŌĆāADHD | 6 (0.9) |
| ŌĆāAutism | 4 (0.6) |
| ŌĆāMeningomyelocele | 2 (0.3) |
| Allergic diseases | 37 (6.0) |
| ŌĆāCowŌĆÖs milk protein allergy | 35 (5.7) |
| ŌĆāFood allergy | 2 (0.3) |
| Endocrine diseases | 19 (3.0) |
| ŌĆāHypothyroidism | 15 (2.4) |
| ŌĆāDiabetes mellitus | 3 (0.5) |
| ŌĆāPanhypopituitarism | 1 (0.2) |
| Gastrointestinal diseases | 17 (2.8) |
| ŌĆāHirschsprung disease | 4 (0.6) |
| ŌĆāAnal stenosis | 3 (0.5) |
| ŌĆāUlcerative colitis | 2 (0.3) |
| ŌĆāCrohn's disease | 2 (0.3) |
| ŌĆāEsophageal atresia | 2 (0.3) |
| ŌĆāPyloric stenosis | 1 (0.2) |
| ŌĆāAnterior displacement of the anus | 1 (0.2) |
| ŌĆāCeliac disease | 1 (0.2) |
| ŌĆāCystic fibrosis | 1 (0.2) |
| Genetic diseases | 8 (1.3) |
| ŌĆāTrisomy 21 | 6 (0.9) |
| ŌĆāVACTERLa) | 1 (0.2) |
| ŌĆāSacral agenesis | 1 (0.2) |
Table┬Ā3.
History and physical examination findings of children with functional versus organic constipation
| History/physical examinationa) | Total (n=616) | Functional (n=511, 83%) | Organic (n=105, 17%) | P valueb) | |
|---|---|---|---|---|---|
| Presenting history | 589 (95.6) | ||||
| Hard stool consistency | 543 (92.2) | 450 (88.1) | 93 (88.6) | 0.233 | |
| Recurrent abdominal pain | 227 (38.5) | 208 (40.7) | 19 (18.1) | 0.309 | |
| Straining | 123 (20.8) | 106 (20.7) | 17 (16.2) | 1.000 | |
| Vomiting | 97 (16.5) | 73(14.3) | 24 (22.8) | 0.181 | |
| Abdominal pain related to defecation | 72 (12.2) | 68 (13.3) | 4 (3.8) | 0.638 | |
| Soiling | 58 (9.8) | 51 (9.9) | 7 (6.7) | 1.000 | |
| Rectal bleeding | 51 (8.7) | 41 (8.0) | 10 (9.5) | 1.000 | |
| Nausea | 48 (8.1) | 39 (7.6) | 9 (8.6) | 0.367 | |
| Painful defecation | 45 (7.6) | 41 (8.0) | 4 (3.8) | 0.141 | |
| Loss of appetite | 21 (3.6) | 17 (3.3) | 4 (3.8) | 0.521 | |
| Recurrent urinary tract infections | 18 (3) | 16 (3.1) | 2 (1.9) | 1.000 | |
| Mucous with stool | 18 (3) | 9 (1.8) | 9 (8.6) | 0.041 | |
| Colic | 14 (2.4) | 7 (1.4) | 7 (6.7) | 0.119 | |
| Positive physical finding | |||||
| Abdominal distension | 56 (9.1) | 46 (9.0) | 10 (9.5) | 0.751 | |
| Perianal fissure | 33 (5.4) | 30 (5.9) | 3 (2.9) | 1.000 | |
| Perianal skin tag | 4 (0.6) | 4 (0.8) | 0 (0) | 1.000 | |
Table┬Ā4.
Medical therapy prescribed for children with functional versus organic constipation
| Laxative type | Total (n=616) | Functional (n=511, 83%) | Organic (n=105, 17%) | P valuea) |
|---|---|---|---|---|
| Lactulose | 395 (64.1) | 325 (63.6) | 70 (66.7) | 0.578 |
| Magnesium hydroxide | 265 (43) | 223 (43.6) | 42 (40) | 0.518 |
| Glycerin suppository | 255 (41.4) | 215 (42.07) | 40 (38.09) | 0.514 |
| Sodium phosphate | 145 (23.5) | 125 (24.4) | 20 (19.04) | 0.258 |
| Bisacodyl | 140 (22.7) | 124 (24.2) | 16 (15.2) | 0.054 |
| Polyethylene glycol | 38 (6.1) | 27 (5.2) | 11 (10.4) | 0.071 |
| Castor oil | 35 (5.7) | 31 (6.1) | 4 (3.8) | 0.489 |
References
1. Kocaay P, E─¤r─▒ta┼¤ O, Dalgi├¦ B. Normal defecation pattern, frequency of constipation and factors related to constipation in Turkish children 0-6 years old. Turk J Gastroenterol 2011;22:369ŌĆō75.


2. Fujitani A, Sogo T, Inui A, Kawakubo K. Prevalence of functional constipation and relationship with dietary habits in 3- to 8-year-old children in Japan. Gastroenterol Res Pract 2018;2018:3108021.




3. Bansal R, Agarwal KA, Chaudhary RS, Sharma M. Clinical manifestations and etiology of pediatric constipation in North India. Int J Sci Stud 2016;4:185ŌĆō90.
4. Khalil MM. Management of childrenŌĆÖs constipation: effect of adjunct physical activity and behavior modifications. J Fam Med Community Health 2015;2:1041.
5. Kondapalli CS, Gullapalli S. Constipation in children: incidence, causes in relation to diet pattern and psychosocial aspects. Int J Contemp Pediatrics 2018;5:6ŌĆō13.


6. Rajindrajith S, Devanarayana NM, Crispus Perera BJ, Benninga MA. Childhood constipation as an emerging public health problem. World J Gastroenterol 2016;22:6864ŌĆō75.



7. Ip KS, Lee WT, Chan JS, Young BW. A community-based study of the prevalence of constipation in young children and the role of dietary fibre. Hong Kong Med J 2005;11:431ŌĆō6.

8. Dehghani SM, Kulouee N, Honar N, Imanieh MH, Haghighat M, Javaherizadeh H. Clinical manifestations among children with chronic functional constipation. Middle East J Dig Dis 2015;7:31ŌĆō5.


9. Altamimi E. Clinical characteristics of pediatric constipation in South Jordan. Pediatr Gastroenterol Hepatol Nutr 2014;17:155ŌĆō61.



10. Hasosah M, Telmesani A, Al-Binali A, Sarkhi A, Alghamdi S, Alquair K, et al. Knowledge and practice styles of pediatricians in Saudi Arabia regarding childhood constipation. J Pediatr Gastroenterol Nutr 2013;57:85ŌĆō92.


11. Appak YC, Sapmaz ┼×Y, Do─¤an G, Herdem A, ├¢zyurt BC, Kas─▒rga E. Clinical findings, child and mother psychosocial status in functional constipation. Turk J Gastroenterol 2017;28:465ŌĆō470.


12. Madhu BS, Agarwal A. Prevalence of chronic constipation and its psychosocial impact on children aged between 4 to 14 years and their parents: a hospital based cross-sectional study. J Evol Med Dent Sci 2015;4:12031ŌĆō42.

13. Ali MW, Sabir OM, Gadour MOE. Pattern and clinical presentation of constipation in children in Sudan. Sudan J Med Sci 2012;7:229ŌĆō32.
14. Biggs WS, Dery WH. Evaluation and treatment of constipation in infants and children. Am Fam Physician 2006;73:469ŌĆō77.

15. Turco R, Miele E, Russo M, Mastroianni R, Lavorgna A, Paludetto R, et al. Early-life factors associated with pediatric functional constipation. J Pediatr Gastroenterol Nutr 2014;58:307ŌĆō12.


16. Joinson C, Grzeda MT, Von Gontard A, Heron J. Psychosocial risks for constipation and soiling in primary school children. Eur Child Adolesc Psychiatry 2019;28:203ŌĆō10.




17. Talachian E, Fereshtehnejad S, Bidari A, Behpour M. The frequency of constipation and it's causes in Iranian children. Med J Islam Repub Iran 2009;23:154ŌĆō9.
18. Veugelers R, Benninga MA, Calis EA, Willemsen SP, Evenhuis H, Tibboel D, et al. Prevalence and clinical presentation of constipation in children with severe generalized cerebral palsy. Dev Med Child Neurol 2010;52:216ŌĆō21.

19. Rome Foundation. Rome IV criteria [Internet]. Rome Foundation; 2016 [cited 2022 Jul 21]. Available from: https://theromefoundation.org/rome-iv/rome-iv-criteria/.
20. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-forlength, weight-for-height and body mass index-for-age: methods and development. Geneva (Switzerland): World Health Organization, 2006:312.
21. De Onis M, Onyango AW, Porghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth references for school-aged children and adolescents. Bull World Health Organ 2007;85:660ŌĆō7.



22. MSD Manual Professional Version. Constipation in children [Internet]. Rahway (NJ): Merck & Co, Inc; 2022 [cited 2022 Aug 1]. Available from: https://www.msdmanuals.com/professional/pediatrics/symptoms-in-infants-and-children/constipation-in-children.
23. Ma Y, Shen Y, Liu X. Functional constipation and bladder capacity and severity of enuresis in children: a correlation study. Int J Clin Exp Med 2018;11:806ŌĆō11.
24. Haghighat M, Amiri Z, Dehghani SM, Safarpour AR, Ataollahi M, Mani A, et al. Investigation of demographic and clinical characteristics of children with constipation referring to the pediatric gastrointestinal clinic, Shiraz in 2014 - 2016. Shiraz E-Med J 2018;19:e13669.

25. Park M, Bang YG, Cho KY. Risk factors for functional constipation in young children attending daycare centers. J Korean Med Sci 2016;31:1262ŌĆō5.




26. American College of Gastroenterology. Constipation and defecation problems [Internet]. North Bethesda (MD): American College of Gastroenterology; 2022 [cited 2022 Aug 12]. Available from: https://gi.org/topics/constipation-and-defection-problems/.
27. Par├® P, Bridges R, Champion MC, Ganguli SC, Gray JR, Irvine EJ, et al. Recommendations on chronic constipation (including constipation associated with irritable bowel syndrome) treatment. Can J Gastroenterol 2007;21 Suppl B(Suppl B): 3BŌĆō22B.

28. Sinha A, Mhanna M, Gulati R. Clinical characteristics of children needing inpatient treatment after failed outpatient treatment for fecal impaction. Pediatr Gastroenterol Hepatol Nutr 2018;21:196ŌĆō202.




29. Kamer B, D├│┼éka E, Pyziak K, Blomberg A. Food allergy as a cause of constipation in children in the first three years of life - own observations. Med Wieku Rozwoj 2011;15:157ŌĆō61.

30. Borrelli O, Barbara G, Di Nardo G, Lucarelli S, Frediani T, et al. Neuroimmune interaction and anorectal motility in children with food allergy-related chronic constipation. Am J Gastroenterol 2009;104:454ŌĆō63.


31. Chumpitazi CE, Henkel EB, Valdez KL, Chumpitazi BP. Soap suds enemas are efficacious and safe for treating fecal impaction in children with abdominal pain. J Pediatr Gastroenterol Nutr 2016;63:15ŌĆō8.



32. Nationwide ChildrenŌĆÖs Hospital. Sickle cell patients should be better monitored for constipation prevention [Internet]. Columbus (OH): Nationwide ChildrenŌĆÖs Hospital; 2010 [cited 2022 Sep 23]. Available from: https://www.nationwidechildrens.org/newsroom/news-releases/2010/06/sickle-cell-patients-should-be-better-monitored-forconstipation-prevention.
33. Yibirin M, De OD, Valera R, Plitt AE, Lutgen S. Adverse effects associated with proton pump inhibitor use. Cureus 2021;13:e12759.



34. Prescribers' Digital Reference. Aluminum hydroxide - drug summary [Internet]. Whippany (NJ): Prescribers' Digital Reference; 2022 [cited 2022 Sep 23]. Available from: https://www.pdr.net/drug-information/aluminum-hydroxide?druglabelid=2835.
35. Santos JD, Lopes RI, Koyle MA. Bladder and bowel dysfunction in children: An update on the diagnosis and treatment of a common, but underdiagnosed pediatric problem. Can Uro Assoc J 2017;11(1-2Suppl 1): S64ŌĆō72.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


