Article Contents
| Clin Exp Pediatr > Volume 65(6); 2022 |
|
Abstract
Background
Purpose
Methods
Results
Supplementary materials
Supplementary material 1
Acknowledgments
Fig. 1.
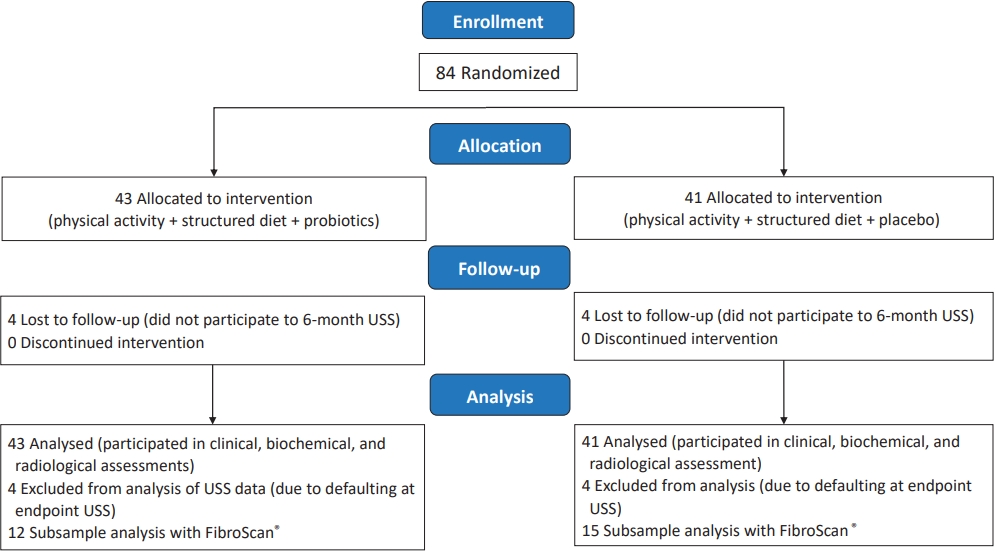
Table 1.
| Parameter | Probiotic group (n=43) | Placebo group (n=41) | P valuea) |
|---|---|---|---|
| Age (yr) | 11.28±1.87 | 12.05±1.45 | NS |
| Sex | NS | ||
| Male | 29 (67.4) | 33 (80.5) | |
| Female | 14 (32.6) | 8 (19.5) | |
| Pubertal status | NS | ||
| Prepubertal | 8 (18.6) | 5 (12.2) | |
| Stage 1 | 14 (32.6) | 11 (26.8) | |
| Stage 2 | 9 (20.9) | 14 (34.1) | |
| Stage 3 | 4 (9.3) | 5 (12.2) | |
| Stage 4 | 1 (2.3) | 3 (7.3) | |
| Liver USS | NS | ||
| Stage I | 39 (90.7) | 38 (92.7) | |
| Stage I–II | 2 (4.7) | 1 (2.4) | |
| Stage II | 2 (4.7) | 2 (4.9) | |
| BMI SDS [28] | 2.56±0.57 | 2.63±0.513 | NS |
| Height SDS [28] | 0.39±0.89 | 0.25±1.10 | NS |
| WC SDS [29] | 2.86±0.50 | 2.91±0.46 | NS |
| SBP SDS [30] | -1.61±1.00 | -1.55±0.92 | NS |
| DBP SDS [30] | 0.84±0.75 | 1.15±0.80 | NS |
| Fat percentage | 41.70±4.68 | 42.2±5.87 | NS |
| FBS (mg/dL) | 75.59±11.79 | 78.35±14.10 | NS |
| OGTT (mg/dL) | 107.85±18.28 | 101.77±20.79 | NS |
| Fasting insulin | 12.23±10.45 | 12.77±8.46 | NS |
| 2-Hour insulin | 93.56±80.91 | 80.49±68.77 | NS |
| TC (mg/dL) | 203.84±41.83 | 190.32±44.36 | NS |
| TG (mg/dL) | 125.47±52.52 | 123.86±58.38 | NS |
| HDL (mg/dL) | 48.97±9.25 | 45.36±8.54 | NS |
| LDL (mg/dL) | 129.55±37.47 | 118.67±33.57 | NS |
| AST (U/L) | 37.87±26.81 | 48.42±57.25 | NS |
| ALT (U/L) | 52.32±34.01 | 60.36±52.29 | NS |
| AST/ALT (U/L) | 0.74±0.17 | 1.79±6.66 | NS |
| GGT (U/L) | 28.42±27.19 | 25.88±12.86 | NS |
| ALP (U/L) | 478.74±205.86 | 484.62±237.33 | NS |
| Albumin (g/L) | 43.44±3.05 | 43.06±3.15 | NS |
| CRP (mg/dL) | 3.00±2.69 | 3.12±2.71 | NS |
Values are presented as mean±standard deviation or number (%).
Pubertal status was not recorded for 7 patients in probiotic group and 3 patients in placebo group.
USS, ultrasound scan; BMI, body mass index; SDS, standard deviation; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; OGTT, oral glucose tolerence test; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TC, total Cholesterol; TG, triglyceride; LDL, Low density lipoprotein; HDL, Hi density lipoprotein; ALP, alkaline phosphate; CRP, C-reactive protein; GGT, gamma glutamyl transferase; NS, nonsignificant.
Table 2.
| Parameter |
Probiotic group (n=43) |
Placebo group (n=41) |
P value† | ||||
|---|---|---|---|---|---|---|---|
| Baseline | End of 6 months | P value* | Baseline | End of 6 months | P value* | ||
| Height SDS | 0.35 (-0.36 to 1.03) | 0.09 (-0.52 to 0.87) | 0.000 | 0.11 (-0.33 to 0.79) | -0.05 (-0.57 to 0.67) | 0.000 | 0.428 |
| BMI SDS | 2.51 (2.09–2.92) | 2.39 (2.024–2.90) | 0.023 | 2.61 (2.24–2.97) | 2.43 (2.17–2.88) | 0.001 | 0.387 |
| WC SDS | 2.86 (2.51–3.31) | 2.85 (2.41–3.16) | 0.262 | 2.92 (2.63–3.2) | 2.84 (2.59–3.13) | 0.690 | 0.778 |
| Fat percentage | 42.3 (38.3–45.0) | 41.7 (37.15–44.85) | 0.196 | 42.6 (37.8–46.5) | 41.8 (36.9–45.55) | 0.026 | 0.700 |
| SBP SDS | -1.99 (-2.42 to -1.08) | -1.5 (-2.37 to -1.1) | 0.345 | -1.46 (-2.41 to -1.13) | -1.35 (-1.65 to -0.42) | 0.226 | 0.219 |
| DBP SDS | 0.44 (0.43–1.58) | 0.44 (0.44–1.58) | 0.274 | 1.52 (0.44–1.58) | 1.57 (0.44–1.58) | 0.000 | 0.050 |
| FBS | 72.7 (66.7–84.6) | 72.9 (67.95–79.45) | 0.658 | 80.1 (68.52–87.75) | 75.75 (70.1–85.55) | 0.570 | 0.379 |
| OGTT | 106.7 (92.3–121.75) | 98.75 (84.65–109.6) | 0.065 | 101 (90.0–121.4) | 96.75 (90.43–120.95) | 0.681 | 0.394 |
| Fasting insulin | 10.3 (4.93–17.25) | 10.4 (4.65–16.19) | 0.673 | 9.86 (6.6–19.0) | 9.6 (7.53–15.4) | 0.779 | 0.751 |
| 2-Hour insulin | 65.65 (41.7–106.17) | 61.7 (29.65–97.1) | 0.187 | 64.3 (27.6–95.3) | 44.5 (16.4–90.1) | 0.202 | 0.167 |
| TC | 197 (180.45–233.5) | 194.4 (168.3–227.6) | 0.399 | 181.9 (158.95–229.5) | 195.2 (156.5–220.4) | 0.943 | 0.436 |
| TG | 120 (85.9–140.4) | 116 (79–142.8) | 0.699 | 107 (87–139.8) | 90 (74.75–121.7) | 0.004 | 0.081 |
| HDL | 46.6 (42.75–54.95) | 50.2 (43.5–56.8) | 0.882 | 45.7 (40.5–50.85) | 48.1 (41.7–54.65) | 0.067 | 0.438 |
| LDL | 120.7 (97.4–160.1) | 120.7 (99.3–152.8) | 0.236 | 115.3 (92.2–149.9) | 119.6 (94.4–145.15) | 0.704 | 0.613 |
| AST | 32.9 (21.25–43.5) | 29.7 (22.75–40.5) | 0.607 | 30.4 (22.35–53) | 25.2 (21.1–36.9) | 0.014 | 0.367 |
| ALT | 44 (29.875–62.25) | 36.4 (23.92–52.97) | 0.199 | 43 (25.7–76.25) | 24 (17.9–49.2) | 0.000 | 0.078 |
| AST/ALT | 0.78 (0.63–0.86) | 0.84 (0.63–0.98) | 0.081 | 0.75 (0.56–0.87) | 0.896 (0.69–1.19) | 0.000 | 0.148 |
| GGT | 22.4 (16.32–27.52) | 22.95 (17.05–32) | 0.135 | 22.5 (17.9–26.5) | 22.2 (17.8–28.5) | 0.845 | 0.910 |
| ALP | 423.3 (297.0–663.8) | 577.9 (419.6–671.9) | 0.158 | 434.8 (291.5–633.45) | 560.5 (426.3–760.2) | 0.014 | 0.576 |
| Albumin | 43 (41.9–45.6) | 42.9 (41.8–44.7) | 0.686 | 42.7 (41.5–45.1) | 43.1 (40.7–44.4) | 0.173 | 0.708 |
| CRP | 2.2 (0.9–3.9) | 1.6 (1–3.2) | 0.527 | 2.4 (1.1–4.0) | 2.1 (1.05–5.25) | 0.369 | 0.363 |
Values are presented as median (interquartile range).
SDS, standard deviation; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; OGTT, oral glucose tolerence test; TC, total Cholesterol; TG, triglyceride; HDL, Hi density lipoprotein; LDL, Low density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; ALP, alkaline phosphate; CRP, C-reactive protein.
Table 3.
Table 4.
| Parameter | Probiotic group (n=12) | P value* | Placebo group (n=15) | P value* | P value† |
|---|---|---|---|---|---|
| Fibroscan CAP baseline | 302 (289–331) | 0.959 | 291.5 (263–319) | 0.638 | 0.572 |
| Fibroscan CAP 6 months | 295 (262–321) | 287 (267–319) | 0.880 | ||
| Fibroscan E baseline | 5.5 (5.3–5.9) | 0.474 | 5.3 (4.8–6.4) | 0.722 | 0.599 |
| Fibroscan E 6 months | 5.8 (4.7–6.8) | 5.2 (4.5–6.8) | 0.914 | ||
| Fibroscan CAP difference | 0 (-15.8 to 32.5) | 0 (-20 to 9) | 0.792 | ||
| Fibroscan E difference | -0.15 (-0.3 to 1.35) | 0 (-0.8 to 0.9) | 0.719 |





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


