Introduction
Cardiac catheterization and intervention in pediatric cardiac diseases have evolved significantly since the publication of previous review articles on this subject in 1992, 1993, 2003, and 2006 in Korea1,2,3,4). During the last 10 years, there have been major technological achievements in pediatric interventional cardiology. There have also been substantial advancements in cardiac imaging modalities, such as intracardiac echocardiography (ICE), real-time 3-dimensional (3D) transesophageal echocardiography (TEE), cardiac computed tomography (CT) and magnetic resonance imaging (MRI), rotational angiography with 3D roadmap, holography, 3D printing, and EchoNavigator and VesselNavigator systems. As a result of such technological advances, more types of congenital heart diseases (CHDs) can be treated in the cardiac catheter laboratory today than ever before. Therefore, the possibility exists that many surgical procedures will be replaced by catheter-based procedures. Furthermore, lesions previously considered resistant to interventional therapies can now be managed with a high success rate, including peripheral pulmonary artery (PA) stenosis, obstructive surgical conduits, and acute postoperative stenosis5). The hybrid approach6,7,8,9,10,11,12,13,14,15) has enabled overcoming the limitations inherent to percutaneous access, expanding the application of endovascular therapies as adjunct to surgical interventions to improve patient outcomes and minimize invasiveness. Some technologies that were considered far-off have actually came into being and emerged as alternative successful therapies, such as percutaneous pulmonic valve implantation16,17,18,19,20,21,22). However, most of the current recommendations23) (including class I recommendations) refer to off-label use of devices that are approved for other coronary, peripheral vascular, or noncardiovascular (typically adult) indications24). Of 126 recommendations23), 56% were based on level C evidence (consensus opinion), 42% were based on level B evidence, and <2% were based on level A evidence. Unfortunately, studies testing the safety and efficacy of catheterization and transcatheter therapy are rare in the field because of the difficulty in identifying a control population, the relatively small number of pediatric patients with CHD, and the broad spectrum of clinical expression. This has resulted in the almost exclusively ŌĆśoff-labelŌĆÖ use of transcatheter devices, initially developed for the management of adult diseases, in the treatment of CHD23). Nevertheless, the pediatric interventional cardiology community has continued to develop less invasive solutions for congenital heart defects to minimize the need for open heart surgery and optimize overall outcomes. For most such interventions, these efforts lead to more highly acceptable outcomes and lower complication rates than the alternatives of surgery or no intervention5). In the following sections, various interventional procedures in patients with CHD are reviewed, and current issues on challenges and limitations in pediatric cardiac interventions are explored.
Defect closure
1. Atrial septal defect
Atrial septal defect (ASD) is the second most common congenital heart defect in adults after bicuspid aortic valve disease. Although most patients with ASD show no significant symptoms, defect closure is recommended in patients with a pulmonary-to-systemic flow ratio of 1.5:1.0 or greater. However, in view of the almost nonexistent surgical mortality in patients with an uncomplicated defect, the potential cardiomyopathic effect of chronic volume overload, the potential risk of paradoxical embolization, and the possibility of a late increase in shunt size owing to the onset of left ventricular disease, even smaller defects may warrant closure.
Since King and Mill invented the first device for closing ASDs in 1976, several devices have been introduced for this purpose. However, only the Amplatzer septal occluder (ASO; St Jude Medical, St. Paul, MN, USA) has been widely used owing to its advantage of being a double disc with a self-centering mechanism. ASO is the first device to receive approval for clinical use in patients with ASD from the U.S. Food and Drug Administration (FDA)25).
ASO can be used in any patient with an ostium secundum ASD with an adequate rim (>5 mm); however, the presence of an anterior rim is not essential. The procedure is usually done under general anesthesia if TEE is planned. However, only minimal sedation is needed under ICE guidance by using the AcuNav catheter (Biosense Webster Co., Diamond Bar, CA, USA).
Complications with the use of ASO are rare, and most of them occur in the immediate period after implantation. Arrhythmia such as atrial flutter or supraventricular tachycardia can be encountered; however, most of them are transient. The other major complication is device embolization, which was reported to occur in 1%, and the most serious complication is development of erosion, which was reported to occur in 0.1%. Amin et al.25) initially suggested that a deficient anterior or superior rim and an oversized device might be risk factors that significantly increases the erosion risk; however, they later modified their proposed risk factors to the following: absence of an aortic rim in multiple views, poor posterior rim consistency, septal malalignment, and dynamic ASD26). Although the exact mechanism of erosion with the use of ASO is still unknown, it is interesting that erosion is very rare or absent with the use of Figulla Flex II (Occlutech Co., Helsingborg, Sweden), Cocoon ASD occluder (Vascular Innovations Co., Nonthaburi, Thailand), or several Chinese ASD occluders that have a similar shape to the original ASO but are softer.
2. Patent ductus arteriosus (PDA)
Since Porstmann first attempted transcatheter closure of patent ductus arteriosus (PDA) in 1967, many devices have been introduced for percutaneous PDA closure. However, the incidence of residual leakage was relatively high (3%ŌĆō38%) and the use of those devices was technically challenging for large PDAs. The Amplatzer duct occluder (ADO, St Jude Medical) has shown excellent results for closing ductus of any size or any shape, except for large tubular ductus that can be closed with the Amplatzer muscular ventricular septal defect (VSD) occluder (St Jude Medical). PDAs may become markedly dilated and aneurysmal, and calcified and degenerative changes within their wall occur in elderly patients, increasing the risk for surgical PDA ligation. Short and wide PDAs present a somewhat higher intraoperative risk. Ligation and division of these PDAs should be performed with cardiac pump on standby. However, the most distinguishing advantage of ADO compared with surgery is that dilated, aneurysmal, calcific, or friable PDAs in older adults can be closed easily with ADO. Nowadays, the percutaneous closure method is the treatment of choice for PDA. Nevertheless, it is still challenging to percutaneously close PDAs in premature babies or small infants. Recently, Amplatzer vascular plug, ADO II, and ADO II additional sizes (St Jude Medical) have been tried for such purpose and showed acceptable results27,28,29,30,31). However, the risk of migration of the device is a problem. Another important issue is closing a large ductus that cannot be closed with a standard-sized ADO. Amplatzer muscular VSD occluder32,33,34,35,36) has been used for this purpose. In some countries, devices similar to the ADO are available in larger sizes (up to 26 mm)37), which can be used to close PDAs >12 mm. There have been case reports of large PDAs with pulmonary hypertension that were closed with an ASO with acceptable results37,38,39,40,41,42).
3. Ventricular septal defect
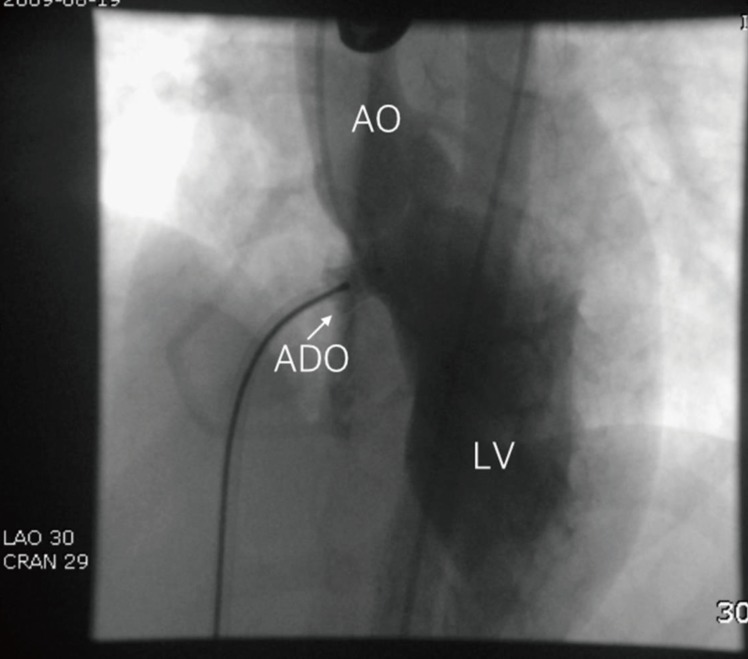
VSD is the most common CHD and perimembranous VSD (PMVSD) is the most common subtype. Surgical closure has been a standard treatment method for PMVSD. Transcatheter closure of PMVSD is a challenging procedure, and the use of an Amplatzer PMVSD occluder (APMVSDO) was first reported in 200343). In several reports, the APMVSDO has been shown to be safe and effective in closing PMVSDs44,45,46,47,48,49). However, several studies reported a high incidence of complete atrioventricular block (CAVB). Researchers from Toronto reported a 22% rate (4 of 20 patients) of CAVB after percutaneous closure of PMVSD with APMVSDO, and most other reports demonstrated an around 5% rate of CAVB50,51,52,53,54). Concerning the mechanism of occurrence of CAVB after percutaneous closure of PMVSDs with APMVSDO, it was suggested that the device may cause direct compression trauma or provoke inflammatory reaction or scar formation in the conduction tissue51). In addition, as the APMVSDO itself has a high stiffness, use of an oversized device would give the appearance of ŌĆśstentingŌĆÖ the VSD and may be more traumatic to the conduction tissue. Compared with the APMVSDO, the Chinese PMVSDO is less stiff and has a longer waist. Therefore, it would theoretically cause less squeezing and less trauma to the conduction tissue even with oversized devices. Liu et al.55) reported a 1.9% (11 of 576) incidence of CAVB. Of these, nine patients recovered in 3 weeks and permanent pacemakers were implanted in 2 patients. Recently, the second-generation APMVSDO has been developed by Saint-Jude Company and clinical trials are being conducted56). Lee et al.57) reported transcatheter closure of PMVSD with ADO in 20 patients with PMVSD. ADO has a short rim (2ŌĆō3 mm) in the distal part, which is supposed to be implanted in the space between the right coronary cusp and the noncoronary cusp of the aortic valve; thus, it is not likely to touch the aortic valve and is safe from aortic regurgitation. Furthermore, it has less contact with the left bundle branch compared with APMVSDO owing to its shorter rim. The other crucial advantage of ADO is that it does not have a proximal disc and thus does not squeeze the His bundle. These are the reasons for the author's preference in using ADO in percutaneous closure of PMVSDs (Fig. 1). However, percutaneous closure of PMVSDs is not approved by the Korean FDA.
Dilatation
1. Pulmonary valvular stenosis
Percutaneous transcatheter valvuloplasty is a treatment of choice for pulmonary valvular stenosis in both children and adults. It is reasonable to dilate valves with a peak gradient of at least 30 mmHg. Adult patients with pulmonary valvular stenosis have large valve annulus and sometimes require the double-balloon technique. The restenosis rates are low58).
2. Aortic valvular stenosis
The congenital bicuspid aortic valve often becomes progressively stenotic; therefore, congenital aortic stenosis more commonly manifests clinically in old age. Balloon valvuloplasty can be a suitable palliation method for children with moderate to severe aortic stenosis (peak-peak gradient >50 mmHg) and mild aortic regurgitation at most, but not for adult patients because of high restenosis rates59). Infants with critical valvular aortic stenosis constitute a special group that needs a different approach. These patients usually show congestive heart failure early in life and rapidly progress to life-threatening conditions. The pressure gradient through the aortic valve is usually <30 mmHg because of poor left ventricular function. Urgent balloon valvotomy under ventilator care is crucial; however, its reintervention rate is high60).
3. Percutaneous pulmonary valve implantation
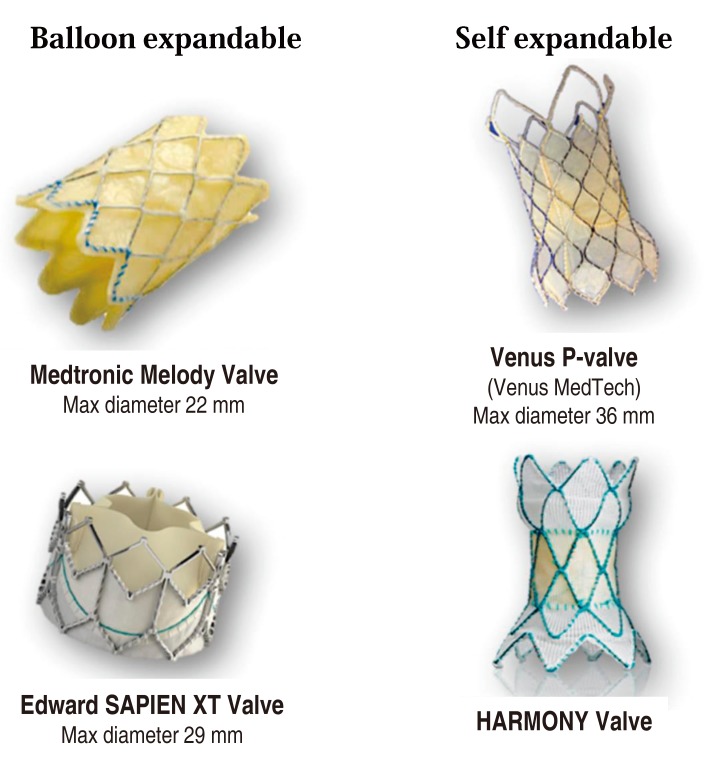
Percutaneous pulmonary valve implantation (PPVI) is an established therapy for pulmonary regurgitation and right ventricular outflow tract (RVOT) obstruction in postoperative patients with tetralogy of Fallot and pulmonary atresia, the Ross procedure for aortic stenosis, truncus arteriosus, and the Rastelli procedure for transposition of the great arteries or double-outlet right ventricle, who had required surgical placement of valved conduits from the right ventricle to the PA18,20,22,61,62,63,64,65,66,67,68,69,70,71,72,73). With time, these conduits become progressively stenotic and insufficient, and surgical replacement of the pulmonary valve has been needed in these cases. PPVI in both animals and humans has become a reality in the last decades18,20,22,61,62,63,64,65,66,67,68,69,70,71,72,73). A percutaneous stent-based expandable pulmonary valve, designed by Dr. Bonhoeffer18), named Melody valve (Medtronic, Minneapolis, MN, USA), uses a glutaraldehyde-treated bovine venous jugular valve sewn into a balloon-expandable stent. This valve has been implanted in more than 10,000 patients; however, this procedure is limited by the conduit size (upper limitation <22 mm) and compliance, patient size (body weight >24 kg needs a 22F sheath), and uncertain long-term efficacy of the valve. In fact, an initial report showed a high incidence of stent fracture22,62). Nowadays, prestenting is a routine procedure before PPVI with the Melody valve to prevent stent fracture68). At present, because of its size, the use of the Melody valve is restricted to only approximately 15% of patients with CHD in whom pulmonary valve replacement is indicated. Extensive research and development are being conducted to design a suitable transcatheter device for patients with a dysfunctional native or patch-augmented RVOT that is too large for the current Melody valve. Further research and development of designs based on this model are under way. The Harmony valve (Medtronic)74), Venus P-valve (Venus Medtech, Shanghai, China)75,76), and Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA)77) were developed and have been used clinically for patients with a large (>22 mm) RVOT (Fig. 2).
Hybrid procedures
The hybrid approach involves the cooperation between surgeons and interventionists to maximize the outcomes and minimize the limitations of their respective approaches. It has enabled overcoming the limitations inherent to percutaneous access, expanding the application of endovascular therapies as adjunct to surgical interventions to improve patient outcomes and minimize invasiveness6,7,8,9,10,11,12,13,14,15).
1. Hybrid procedure for hypoplastic left heart syndrome
The first hybrid palliative approach to hypoplastic left heart syndrome was reported in 1993 with surgical banding of the branch PAs and percutaneous stenting of the arterial duct78,79), and modified by the Gissen group with the goal of achieving distal PA pressure <50% of the systemic pressure and a reduction in systemic oxygen saturation to approximately 80%12), which showed 82% survival through stage II palliation80). Further modification by the Columbus group13), who performed PA banding and stenting of the arterial duct through the PA with the surgeon and interventionist in the same hybrid room, showed 83% survival through stage III palliation81).
2. Hybrid approach to VSD closure (perventricular closure)
The perventricular approach for closure of membranous and muscular VSDs in infants was reported in 199982). The benefits of this approach were direct access to the heart after surgical sternotomy for direct placement of a closure device across the VSD under the guidance of TEE, without the need for cardiopulmonary bypass or radiation8,9).
Fetal intervention
Fetal interventions for CHD have become important treatment modalities in the last 20 years, especially for severe aortic or pulmonary stenosis. Critical aortic stenosis in utero can progress into hypoplastic left heart syndrome, which has a high postnatal mortality rate. Fetal interventions may remodel cardiac morphology and function to such an extent that it may favorably alter the in utero natural history, resulting in improved prenatal and postnatal outcomes, including an increased likelihood of achieving biventricular circulation84). However, in a recent meta-analysis study85), fetal aortic valvuloplasty had a live-birth rate of 65% with a neonatal death rate of 16%. In fetal pulmonary valvuloplasty, the live-birth rate was 56% and the neonatal mortality was 33%. Although the results of the meta-analysis are encouraging in terms of perinatal survival, they should be interpreted with caution when comparing with procedures performed after delivery.
Advances in cardiac imaging
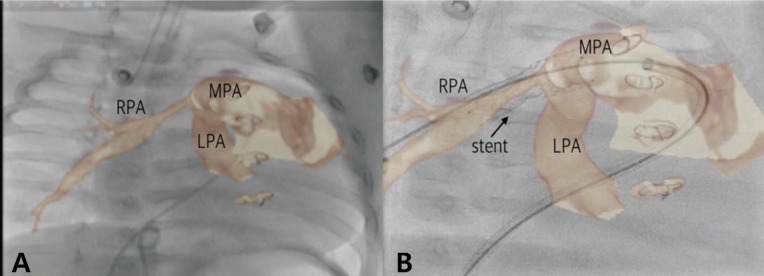
During the last 10 years, development of imaging software has facilitated major advances in cardiac imaging modalities such as ICE, real-time 3D TEE, cardiac CT and MRI, rotational angiography with 3D roadmap, holography, 3D printing, and EchoNavigator and VesselNavigator systems. Especially, fusion imaging with fluoroscopy and echocardiography (EchoNavigator) or fluoroscopy and tomography (VesselNavigator) further assist complex percutaneous interventional catheterization procedures. Cardiac imaging has to be done in real time with an excellent temporal resolution to ensure hand-eye synchronization and accurate positioning between the device and the target area86). As a result of recent technological advances, more types of CHDs than ever before are now possible to treat in the cardiac catheter laboratory. For example, VesselNavigator is a new fusion imaging modality that overlays a 3D CT image onto a live fluoroscopy image (Fig. 3). It provides an intuitive and continuous 3D roadmap by rotating overlaid CT images at any direction, and allows for both advanced diagnostic and interventional cardiac catheterization procedures in patients with CHD. Therefore, lesions previously considered resistant to interventional therapies can now be managed with a high success rate, such as peripheral PA stenosis87).
Evolution and challenges
Several kinds of pediatric cardiac interventional procedures that were considered far-off in the last 2 decades have become standards of care owing to major technological advances, including PPVI. Therefore, the indications of pediatric cardiac interventions should be modified, and Feltes et al.23) summarized the American Heart Association recommendations on the indications for cardiac catheterization and intervention in pediatric cardiac disease, which were endorsed by the American Academy of Pediatrics and Society for Cardiovascular Angiography and Intervention. However, even in the United States, most of the current recommendations about pediatric cardiac interventions (including class I recommendations) refer to off-label use of devices, because it is difficult to study the safety and efficacy of catheterization and transcatheter therapy in pediatric cardiac patients. This difficulty results from the challenge of identifying a control population and the relatively small number of pediatric patients with CHD. In Korea, many kinds of devices for interventional procedures are not available, such as several kinds of stents such as Genesis XD, Valeo, or covered stents. Furthermore, several kinds of interventional procedures are not approved by the Korean FDA, including percutaneous closure of PMVSD, although it has been performed since 2009 and reported by the author and coworkers57). Nevertheless, the pediatric interventional cardiology community has continued to develop less invasive solutions for congenital heart defects to minimize the need for open heart surgery and optimize overall outcomes.
Conclusions
As a result of recent technological advances, more types of CHDs than ever before are now possible to treat in the cardiac catheter laboratory. Furthermore, lesions previously considered resistant to interventional therapies can now be managed with high success rates. In addition, the hybrid approach, fetal cardiac intervention, and percutaneous pulmonic valve implantation have evolved significantly. However, most current recommendations for interventional procedures refer to off-label use of devices. Unfortunately, studies testing the safety and efficacy of transcatheter therapy in pediatric cardiac patients are rare because of the difficulty in identifying a control population, the relatively small number of pediatric patients with CHD, and the broad spectrum of clinical expression. Nevertheless, the pediatric interventional cardiology community has made attempts to develop better solutions to minimize the need for open heart surgery and optimize overall outcomes.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


