< Previous Next >
Article Contents
| Clin Exp Pediatr > Volume 59(8); 2016 |
|
Abstract
Purpose
Venous angioma (VA) is the most common congenital abnormality of the intracranial vasculature. This study aimed to investigate the relationship between VA and epilepsy and to identify the characteristics of children with VA and epilepsy.
Methods
The records of all patients aged less than 18 years who underwent brain magnetic resonance imaging (MRI) at Pusan National University Hospital were retrospectively reviewed. Patients with isolated VA and patients with normal MRI were compared in terms of the prevalence of epilepsy.
Results
In total, 2,385 pediatric patients who underwent brain MRI were enrolled. Isolated VA was identified in 26 patients (VA group). Among the patients with normal MRI findings, 225 age- and sex-matched patients to the VA-group were assigned to the control group. Nine patients in the VA group (9 of 26, 34.6%) and 27 patients in the control group (26 of 225, 11.5%; P<0.001) had epilepsy. In the VA group, 20 patients (76.9%) had the VA in the cerebral hemispheres, and 6 patients (23.1%) had the VA in the brainstem and cerebellum. The latter showed a higher prevalence of epilepsy (5 of 6, 83.3%) than the former (4 of 20, 20.0%; P=0.004). Among the nine patients who had epilepsy with VA, patients whose VA involved the brainstem and cerebellum showed a significantly higher frequency of abnormal Electroencephalographic findings than patients whose VA involved the cerebral hemispheres (P=0.016).
Venous angioma (VA) is a congenital anomaly of the medullary vein, the vessel that drains into the transparenchymal venous stem ("caput medusa" on the venous phase of angiography)1,2). This is also referred to as a developmental venous anomaly3). VA is the most common intracranial vascular malformation in neuroimaging and autopsy series4). Magnetic resonance imaging (MRI) has detected VA in 0.7%–2.5% of all the studies5). The mechanism of VA is still unknown, but intrauterine accidents may be involved in the abnormal formation of the medullary veins or their tributaries6).
VA has been reported as an asymptomatic condition because it is often found incidentally during the evaluation of other different clinical features7). On the contrary, VA has been reported as a cause of seizures with incidence of seizures associated with symptomatic VA ranging from 8%–29%8). However several studies that utilized MRI demonstrated that these symptoms are related to other pathologies such as cavernous angioma and cortical dysplasia, which are highly epileptogenic lesion5). Controversy still exists in the literature regarding the epileptogenesis of VA. This study investigated whether VA is associated with epilepsy by analyzing the prevalence and characteristics of epilepsy associated with intracranial VA in children.
A retrospective study was conducted in children under 18 years old who underwent magnetic resonance (MR) brain between January 2009 and December 2013 in Pusan National University Hospital (n=2,385). The exclusion criteria were as follows: (1) presence of other pathologic brain lesion on MRI, (2) prior diagnosed central nervous system infection, genetic mutation, known syndromic disease, or inborn error of metabolism, (3) history of previous head trauma. The patients who had the appropriate finding of VA were designated as the VA group. Among the patients with normal MRI finding after excluding patients with MRI lesions other than VA, patients with age and sex matched to VA group was assigned to control group.
All children underwent MRI examination on a 1.5- or 3-Tesla Siemens Sonata (Magnetom symphony, Siemens Medical Solutions, Erlangen, Germany) using the standard circularly polarized head coil. Patients who could not undergo MRI test, were sedated with oral chloral hydrate (50 mg/kg), followed by intravenous midazolam (0.1 mg/kg; maximum, 5 mg). The MR protocol included scout pilot scans, followed by an axial 3-dimensional (3D) T1-weighted gradient-echo acquisition (TR/TE=20/5.6 msec; section thickness=2 mm; TR/TE, a basic pulse sequence parameters), an axial T2-weighted turbo spin-echo acquisition (TR/TE=5,020/106 msec; section thickness=4 mm), an axial velocity compensated 3D gradient echo susceptibility weighted imaging acquisition, and a postgadolinium T1-weighted acquisition (imaging parameters were the same as the noncontrast T1 sequence mentioned above). The contrast agent gadolinium-diethylenetriamine pentaacetic acid (Magnevist, Berlex, Wayne, NJ, USA) was bolus injected by a power injector (Medrad, Spectris MR injection system, Pittsburgh, PA, USA) with a dose of 0.1 mmol/kg of body weight at a rate of 3 mL/sec. The two neuroradiologists detected a presence of brain lesion.
Electroencephalograms (EEGs) were recorded using a Grass-Telefactor system (Telefactor Cop., Conshohoken, PA, USA) or Stellate Harmonie EEG (Stellate System Inc., Montreal, QC, Canada) with a high-cut filter 70 Hz for more than 40 minutes during sleep with or without the fully alert period. Patients with difficulty of the cooperation for the EEG examination were sedated with only chloral hydrate (50 mg/kg). The electrodes were applied in accordance to the international 10–20 system, referenced to both ears. A detailed interpretation of the interictal features of the EEG records was performed by one of the board certified clinical epileptologist. A limited interrater agreement study was conducted by another epileptologists with no their clinical history. We reviewed the background and interictal activities of each EEG recording. The medical charts of both groups (VA and control groups) were investigated the mean age, gender, frequency and duration of seizures, seizure types, and final diagnosis. In the VA group, the location of VA, history of epilepsy, and EEG findings were investigated to analyze the relationship between location of VA and seizure activity.
Ethics permission for this study was granted by the Institutional Review Board of Pusan National University Hospital and fully informed written consent was obtained from each participant.
Data were stored in a dedicated access database and verified for accuracy. Statistical analyses were performed with SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) using raw scores. Analyses presented here focus on the comparisons between the 2 groups (VA and control). Statistical comparison of the continuous and categorical variables was tested by means of t test and chi-square test as appropriate for the data. In addition to univariate nonparametric statistical tests, the results of the t test and chi-square tests were confirmed with the Mann-Whitney test and Fisher exact test. A P value <0.05 was regarded as statistically significant.
Twenty-six patients with VA were identified. Among 1,239 patients with normal MRI finding, 225 patients with age and sex matched to VA group were included to control group. There were 9 epilepsy patients in the VA group (9 of 26, 34.6%) and 27 patients in the control group (26 of 225, 11.5%; P<0.001). The prevalence of epilepsy in the VA group was significantly higher than in control group (P<0.001) (Fig. 1). The chief complaints prompting MRI in the VA group were seizure (n=11), headache (n=8), developmental delay (n=1), and others (n=6).
In the 26 patients of the VA group, VA was located in the cerebral hemisphere in 20 patients (76.9%) and in the brainstem and cerebellum in six patients (23.1%) (Table 1). A prevalence of epilepsy was 4 patients with VA located in the cerebral hemisphrere, 2 patients in the brainstem and 3 patients in the cerebellum. A significantly higher prevalence of epilepsy was found in patients with VA located in the cerebral hemisphere (83.3%, 5 of 6) than those in the brainstem and cerebellum (20%, 4 of 20, P=0.004) (Fig. 2).
The clinical characteristics of the nine patients with epilepsy in VA group are summarized in Table 2. The age at onset of epilepsy ranged from 5 months to 13 years. Seven patients had complex partial seizures. The remaining 2 patients had generalized tonic seizures (n=1) and partial seizure with secondary generation (n=1). Among the 9 patients diagnosed with epilepsy, abnormal EEG were exhibited in 4 cases. The location of VA of 4 patients with abnormal EEG were the brainstem in 1 patient (50%, 1 of 2) and the cerebellum in 3 patients (100%, 3 of 3) respectively. All patients with VA located in the cerebral hemisphere had normal EEG findings. The proportion of abnormal EEG finding was significantly higher in VA located in the brainstem and cerebellum (80%, 4 of 5) than in VA located in the cerebral hemispheres (0%, 0 of 4, P=0.016) (Fig. 3). Especially in VA located in the cerebellum, all patients showed abnormal EEG findings.
VA is the most common vascular malformation identified in neuroimaging studies. The detection rate of VA by MRI is variable, with reported rates between 0.7%–2.5%, and is in two third of the cases present supratentorially5,9). The rates of VA and supratentorial involvement in our study are 0.9% (26 of 2,385) and 76.9% (20 of 26), which is similar proportion.
VA might manifest as an asymptomatic and benign condition. Garner et al.10) reviewed a series of 100 patients with radiologically identifiable VA and showed that seizures and focal neurological deficits were unusual associated findings and that the natural history of VA is relatively benign. Another study reported that epileptic seizures may actually originate from areas of cortical dysplasia which are occasionally located near a VA11). On the other hand, VA has been reported as a seizure prone lesion. The relationship between VA and epilepsy has been widely reported in the previous studies, and it has been suggested that 8%–29% of patients with VA are epileptic8). However, there was no mention about the prevalence in the control group and the statistical significance.
Although VA is usually asymptomatic, when symptomatic, the most common symptoms are headaches, seizures, and hemorrhages, and we have occasionally treated the patients with VA associated with epilepsy. Presently, 36% of patients in the VA group and 11.5% of patients in the control group had epilepsy (Fig. 1); the difference was highly significant (P<0.001). This findings are contrary to the view the natural history of VA is a benign without significant clinical importance. Rather, our results support the view that VA is associated with epilepsy.
The anatomical distribution of VA has been reported as predominantly in the supratentorial location (two-thirds) more than in the infratentorial location (one-third)9). This was similar to our results. In 26 patients with VA, the cerebral hemispheres were involved in 20 patients (76.9%) and brainstem and cerebellum were involved in 6 patients (23.1%) (Table 1). Topper et al.9) reported that in patients presenting with seizures a supratentorial location of VA is more frequent than in the remaining patients. We documented that the prevalence of epilepsy was significantly higher in the patients with VA of the brainstem and cerebellum (83.3%, 5 of 6) than in them of the cerebral hemisphere (20%, 4 of 20, P=0.004) (Fig. 2). Our results differed from those of Topper et al.9), who included patients with other intracranial lesions as well as isolated VA. An important finding in our study was that predominance of infratentorial location can be found in patients with isolated VA and epilepsy.
Studies about the role of the brainstem and cerebellum in epileptogenesis are sparse. Traditionally, epileptic seizures have been thought of as cerebrocortical phenomena, but there have been reports of seizures that were thought to commence within the cerebellum12,13,14). Though no evidence exists to suggest that epileptic electrical activity occurs during these seizures, these reports postulate that at least in cases of cerebellar pathology such as cerebellar gangliogliomas, the cerebellum may be an epileptogenic lesion. In addition to its epileptogenic effects, the cerebellum has also been proposed to have a role in inhibition of seizures. Krauss and Koubeissi15) proposed that the cerebellar stimulation could reduce epileptogenic activity because of its gamma-aminobutyric acid-ergic Purkinje cell inhibitory output and widespread cortical projections. Together, nearly 70% of the patients who are reported with cerebellar stimulation in the published literature had reduction in seizures15). Blumenfeld et al.16) suggested the idea that increased cerebellar activity may contribute to seizure termination and/or to postictal suppression. From these results, we can speculate that VA may cause some degree of functional deficit in the cerebellum to inhibit seizure activity, rather than develop epileptogenic lesion by itself.
We examined 9 patients with VA and epilepsy to assess whether there was topographic concordance between the location of VA and ictogenesis in patients with epilepsy. In the interictal EEG, 5 patients had normal findings and 4 patients had abnormal findings. The proportion of abnormal EEG finding was significantly higher in VA located in the brainstem and cerebellum (80%, 4 of 5) than in VA located in the cerebral hemispheres (0%, 0 of 4, P=0.016) (Fig. 3). Especially in VA located in the cerebellum, all patients showed abnormal EEG findings. Thus we founded that convulsion with abnormal EEG finding was frequently associated with VA of the brainstem and cerebellum. However, we cannot know whether abnormal EEG findings in VA located in the brainstem and cerebellum might be due to suppression of the inhibitory role of the cerebellum. Also, this study has some limitations as this study was conducted retrospectively. The number of VA group and control group was quite different, and the number of VA patients was too small compared with control group, limiting the statistical power.
However, our study has important clinical implications. We can suggest that VA might be associated with epilepsy. In addition, seizures were more frequently observed in the VA of the brainstem and cerebellum than in them of the cerebral hemispheres. Abnormal findings in EEG were also more often associated with VA located in the brainstem and cerebellum. The present results can indicate that special attention may be required for patients with VA especially located in the brainstem and cerebellum. Further studies with adequate sample size, and preferably multicenter are needed to investigate the relationship between the location of VA and developing epilepsy.
Acknowledgments
This study was supported by a clinical research grant from Pusan National University Hospital 2015.
Notes
Conflicts of interest:
No potential conflict of interest relevant to this article was reported.
References
1. Kraemer DL, Awad IA. Vascular malformations and epilepsy: clinical considerations and basic mechanisms. Epilepsia 1994;35(Suppl 6): S30–S43.

2. Lasjaunias P. Vascular diseases in neonates, infants and children: interventional neuroradiology management. New York: Springer-Verlag, 1997.
3. Truwit CL. Venous angioma of the brain: history, significance, and imaging findings. AJR Am J Roentgenol 1992;159:1299–1307.


4. Sarwar M, McCormick WF. Intracerebral venous angioma. Case report and review. Arch Neurol 1978;35:323–325.


5. Striano S, Nocerino C, Striano P, Boccella P, Meo R, Bilo L, et al. Venous angiomas and epilepsy. Neurol Sci 2000;21:151–155.


6. Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev 1986;9:233–242.


7. Pereira VM, Geibprasert S, Krings T, Aurboonyawat T, Ozanne A, Toulgoat F, et al. Pathomechanisms of symptomatic developmental venous anomalies. Stroke 2008;39:3201–3215.


8. Fujii M, Kitahara T, Moroi J, Kato S, Ito H. Temporal lobe epilepsy associated with cerebral venous angioma: case report. Neurol Med Chir (Tokyo) 1998;38:413–416.


9. Töpper R, Jurgens E, Reul J, Thron A. Clinical significance of intracranial developmental venous anomalies. J Neurol Neurosurg Psychiatry 1999;67:234–238.



10. Garner TB, Del Curling O Jr, Kelly DL Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg 1991;75:715–722.


11. Gumus A, Yildirim SV, Kizilkilic O, Cengiz N, Cemil T. Case report: seizures in a child caused by a large venous angioma. J Child Neurol 2007;22:787–789.


12. Harvey AS, Jayakar P, Duchowny M, Resnick T, Prats A, Altman N, et al. Hemifacial seizures and cerebellar ganglioglioma: an epilepsy syndrome of infancy with seizures of cerebellar origin. Ann Neurol 1996;40:91–98.


13. Chae JH, Kim SK, Wang KC, Kim KJ, Hwang YS, Cho BK. Hemifacial seizure of cerebellar ganglioglioma origin: seizure control by tumor resection. Epilepsia 2001;42:1204–1207.


14. Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav 2002;3:219–231.


Fig. 1
Comparison of the prevalence of epilepsy between the venous angioma and control groups. *P<0.05.
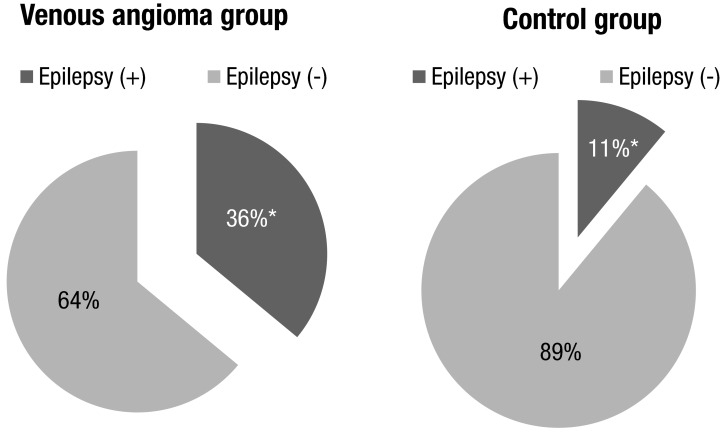
Fig. 2
Comparison of the prevalence of epilepsy according to the location of the venous angioma. *P<0.05.
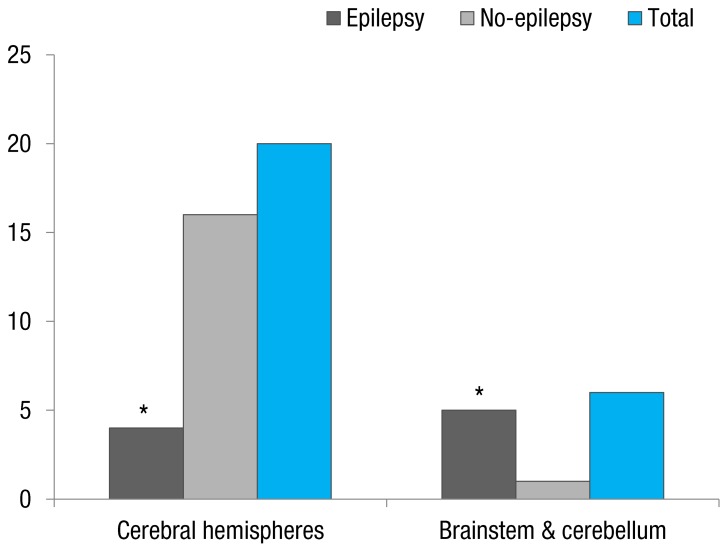
Fig. 3
Comparison of the electroencephalogram (EEG) abnormalities according to the location of the venous angioma. *P<0.05.
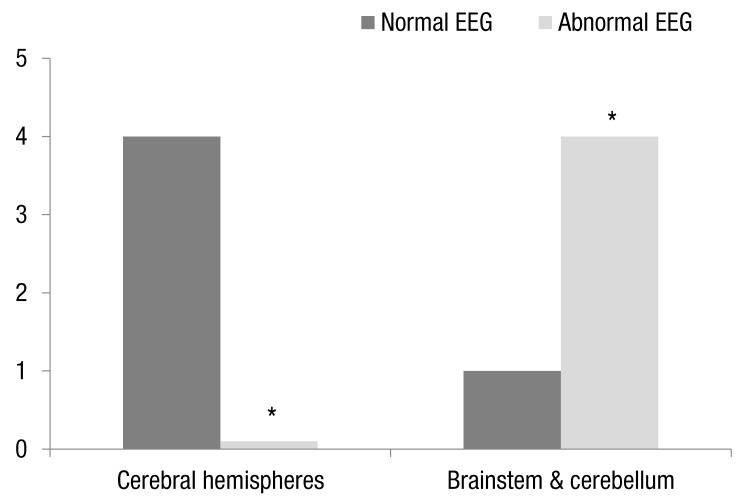
Table 1
Anatomical distribution of venous angioma (n=26)
| Location | No of patients |
|---|---|
| Supratentorial | 20 (76.9) |
| Frontal | 9 |
| Temporal | 2 |
| Parietal | 5 |
| Occipital | 2 |
| Basal ganglia | 2 |
| Infratentorial | 6 (23.1) |
| Cerebellum | 4 |
| Brainstem | 2 |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


