Introduction
Acute diarrhea is a common condition, especially among children, due to age-specific differences in host defense mechanisms1,2,3). Validated laboratory testing for viral, bacterial, and parasitic pathogens is helpful for improving the management of acute diarrhea, and for monitoring and controlling the spread of these infections4). However, diarrheal illnesses remain a leading cause of pediatric morbidity and mortality worldwide. Many of the gastroenteritis cases among young children are caused by viruses that belong to four distinct families: rotavirus, noroviruses, astrovirus, and adenovirus5).
Human rotavirus has been considered the cause of most acute diarrhea cases among infants and young children worldwide, including in Korea6,7,8,9). However, rotaviral enteritis can be prevented via vaccination, and 2 vaccines were introduced in Korea during 2007 (Rota Teq, Merck & Co., Inc., Kenilworth, NJ, USA) and 2008 (Rotarix, GlaxoSmithKline Biologicals, Rixensart, Belgium)8). As these rotavirus vaccines have become more widely available, the detection rate for group A rotavirus has decreased from 12.3% in 2005 to 8.3% in 2008, according to the Korea Centers for Disease Control and Prevention (KCDC) reports10). Though this reduction in the prevalence of rotavirus, human norovirus has recently emerged as an important cause of severe gastroenteritis among children, and has been detected at rates of 3%-30% among hospitalized children in developed and developing countries1,11).
Viral pathogens can lead to outbreaks at a time when clinical services are experiencing peak demand, as well as seasonal outbreaksViral pathogens can lead to outbreaks at a time when clinical services are experiencing peak demand, as well as seasonal outbreaks12). For example, the peak incidence of rotavirus is known to occur between late fall and winter. However, little is currently known regarding the postvaccination prevalence or seasonal changes in viral infections. Because the rotavirus vaccination rate has increased as annually13,14,15), we hypothesized that the prevalence or seasonal change in viral infections might change after vaccination. Furthermore, stool testing provides a relatively easy and simple way to detect viruses using multiplex reverse transcription polymerase chain reaction (multiplex RT-PCR), which is sensitive and specific for detecting rotavirus, norovirus (groups I and II), astrovirus, and group F adenovirus (serotypes 40 and 41)16). Therefore, we aimed to identify the viruses that caused gastroenteritis according to month, in order to determine their relative changes in prevalence. By evaluating the patients' age and clinical characteristics, as well as the periodic distribution of viral gastroenteritis, we hoped to provide information that can help prevent the spread of disease and facilitate prophylaxis and surveillance of acute viral gastroenteritis among children in our region.12). For example, the peak incidence of rotavirus is known to occur between late fall and winter. However, little is currently known regarding the postvaccination prevalence or seasonal changes in viral infections. Because the rotavirus vaccination rate has increased as annually13,14,15), we hypothesized that the prevalence or seasonal change in viral infections might change after vaccination. Furthermore, stool testing provides a relatively easy and simple way to detect viruses using multiplex reverse transcription polymerase chain reaction (multiplex RT-PCR), which is sensitive and specific for detecting rotavirus, norovirus (groups I and II), astrovirus, and group F adenovirus (serotypes 40 and 41)16). Therefore, we aimed to identify the viruses that caused gastroenteritis according to month, in order to determine their relative changes in prevalence. By evaluating the patients' age and clinical characteristics, as well as the periodic distribution of viral gastroenteritis, we hoped to provide information that can help prevent the spread of disease and facilitate prophylaxis and surveillance of acute viral gastroenteritis among children in our region.
Materials and methods
1. Patients
This study evaluated 449 children who visited or admitted the CHA Bundang Medical Center for treatment of acute gastroenteric symptoms including abdominal pain, vomiting, as well as diarrhea for 1 year, from June 2014 to May 2015. Among the 449 stool specimens, we excluded 104 specimens for containing common bacterial pathogens such as Campylobacter spp, Clostridium toxin B, Clostridium perfringens, and Salmonella spp. The remaining 345 specimens were submitted to simultaneous testing for norovirus, rotavirus, astrovirus, and adenovirus via multiplex RT-PCR. The stool samples were collected from patients who were 1 month to 16 years old, and 203 of the 345 patients (58.8%) were male.
2. Viral nucleic acid extraction and multiplex RT-PCR
We collected at least 1 g of diarrhea stool from each patient before they underwent treatment. The collected stool samples were diluted 10× with phosphate buffered saline, centrifuged for 10 minutes at 12,000 rpm, and the supernatant was collected. Viral nucleic acid extraction and cDNA synthesis were performed using a viral DNA/RNA kit (PerkinElmer Inc., Rodgau, Germany) and a cDNA synthesis kit (Thermo Fisher Scientific Inc., Waltham, MA, USA), according to the manufacturers' instructions. For the multiplex RT-PCR, the extracted nucleic acids were mixed with primers and buffers (Seeplex Diarrhea-V ACE detection kit, Seegene Biotechnology Inc., Seoul, Korea), and amplified using a SEEAMP PCR machine (Seegene Biotechnology Inc.). The amplified viral nucleic acid products were loaded onto 2% agarose gel with a DNA size marker (100 bp), a positive control, and a negative control, and were submitted to electrophoresis, according to the manufacturer's instructions. After electrophoresis, he PCR products' sizes were matched to the corresponding product: norovirus GI (NoV-GI, 304 bp), norovirus GII (NoV-GII, 214 bp), group A rotavirus (RotV, 541 bp), enteric adenovirus (AdeV, 411 bp), and astrovirus (AstV, 650 bp).
3. Clinical manifestations
We retrospectively analyzed the clinical characteristics of patients who were positive for the various viruses. The average age, sex ratio, clinical characteristics (e.g., diarrhea, vomiting, fever, abdominal pain or irritability, and dehydration) were compared or each virus. Diarrhea was defined as >3 instances of sudden-onset watery diarrhea within 24 hours. Fever was defined as a body temperature of ≥38.0℃. Dehydrated patient was defined who needs intravenous hydration based on the presence of decreased skin turgor on the abdomen, decreased frequency of urination below 1/3 of daily frequency, or weight loss over 5% of body weight.
4. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics er. 21.0 (IBM Co., Armonk, NY, USA). Continuous variables were compared using one-way analysis of variance, and independent variables were compared using the chi-square test. Differences with a P value of <0.05 were considered statistically significant. This study was approved by the Ethical Committee of the CHA Bundang Medical Center in Seongnam, Korea (approval number: BD2015-008)
Results
Viruses were detected in 157 of the 345 cases (45.5%). The most common virus was norovirus, which was detected in 67 cases (19.4%), followed by rotavirus (45 cases, 13.0%), adenovirus (26 cases, 7.5%), and astrovirus (9 cases, 2.6%) (Table 1). Mixed viral infections were detected in 10 cases (2.9%), including norovirus with adenovirus (2 cases), norovirus with astrovirus (3 cases), norovirus with rotavirus (1 case), adenovirus with astrovirus (2 cases), adenovirus with astrovirus (1 case), and norovirus with rotavirus and astrovirus (1 case).
The mean ages of the infected patients were 2.95±0.46 years for norovirus, 2.19±0.62 years for adenovirus, 2.03±0.30 years for rotavirus, and 1.91±0.48 years for astrovirus. These differences were statistically significant (P=0.012).
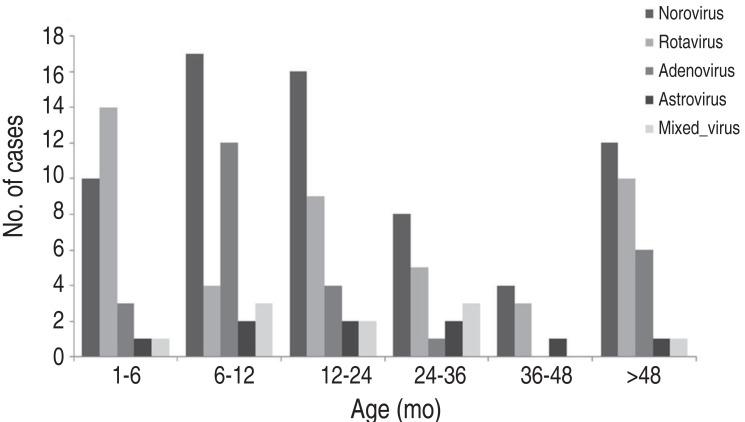
Overall, viral infection occurred mainly between 6-24 months, in 45.2% of cases, after which prevalence decreased. Although norovirus infection occurred at a similar rate among all age groups, it was most frequent at 6-24 months (49.3%). Rotavirus was most prevalent at below 6 months among all age groups. Adenovirus was prevalent at 6-12 months, and astrovirus infection occurred evenly regardless of age (Fig. 1). The male-to-female ratio for viral gastroenteritis was 1:0.70, and there were no statistically significant differences in the ratios for each virus (1:0.77 for norovirus, 1:0.52 for rotavirus, 1:0.3 for adenovirus, and 1:1.25 for astrovirus).
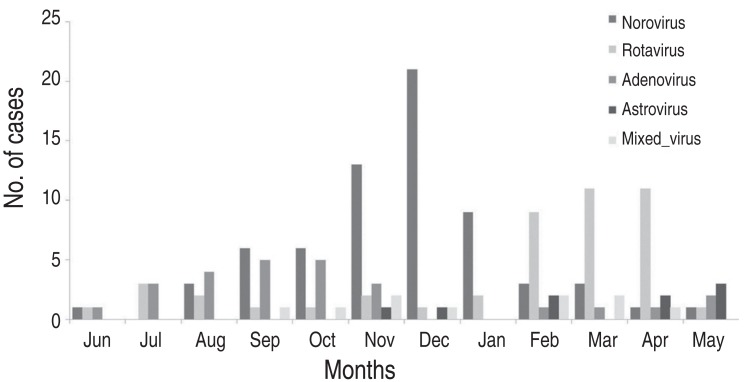
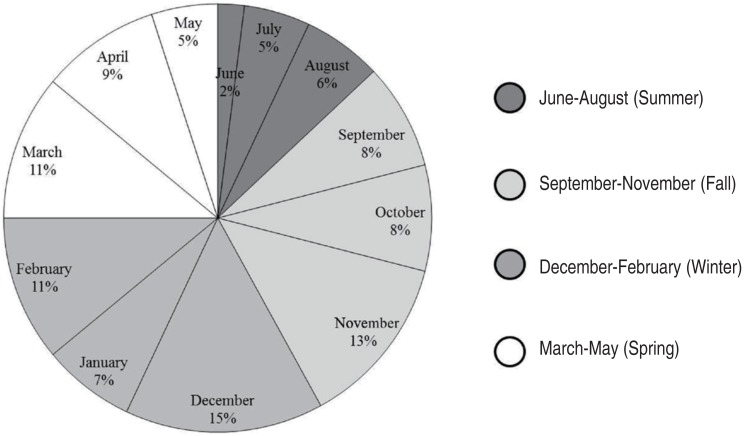
The highest prevalence of viral gastroenteritis was observed in winter between December and February (Fig. 2). The prevalence of norovirus began to increase in November (19.4%) and reached its highest prevalence in December (31.3%), which continued into January (20.9%). In February-April, when the prevalence of norovirus decreased, rotavirus exhibited a trend towards an increasing prevalence (31.1%, 35.6%, and 35.6%, respectively). Adenovirus was most prevalent during summer and early fall (July-October: 11.5%, 15.4%, 23.1%, and 23.1%, respectively), and no cases were observed during December and January. Astroviral gastroenteritis only occurred during winter and spring (November.May), and did not occur between June and September (Fig. 3).
The characteristic symptoms of acute viral gastroenteritis were diarrhea (61.1%), vomiting (57.3%), fever (47.8%), and abdominal pain (38.2%). Diarrhea was the most common for astrovirus, which was followed by rotavirus and adenovirus, although vomiting did not exhibit any pathogen-specific differences. Fever was the most frequent for rotavirus and astrovirus, followed by norovirus and adenovirus. Abdominal pain or irritability was the most commonly experienced by patients with a noroviral infection, although this increase was not statistically significant. Dehydration was shown the most frequently among patients with rotaviral infection (33.3%). Norovirus, which had the highest prevalence in all patients, did not frequently exhibit symptoms of severe dehydration (12.1%, P=0.044). Most patients with mixed infections exhibited clinical symptoms that were similar to those of the patients with single infection (Table 2).
Discussion
Diarrhea is a common condition that causes approximately 19% of all child deaths worldwide, with each child worldwide experiencing an average of 3.2 diarrhea episodes per year17,18,19). Thus, ongoing studies are evaluating the management and prevention of acute pediatric gastroenteritis10). Recently, socioeconomic development, increased awareness of personal hygiene, and the commercialization of rotavirus vaccines has changed the prevalence of viral gastroenteritis2,12,20).
Although noroviruses are considered the leading cause of foodborne diseases and nonbacterial gastroenteritis worldwide, the KCDC have reported that the most commonly detected virus was rotavirus (15.4%) during 201114). However, those results were from the entire region of South Korea, and it is possible that areas with high vaccination rates may provide different results. Moreover, recent studies have used more sensitivity and specific diagnostic methods for detecting rotavirus (e.g., RT-PCR or an immunochromatographic assay), compared to electron microscopy or enzyme-linked immunosorbent assay, which may have provided more accurate diagnoses21,22). We also used multiplex RT-PCR to simultaneously detect rotavirus, norovirus (genogroups I and II), astrovirus, and adenovirus in stool specimens from children with acute diarrhea.
In the present study, we found that 45.5% of all stool samples were positive for viruses, which is within the 31.5%-59% range and is roughly similar to previously reported studies10,23,24). The most common virus pathogen was norovirus, although that finding contradicts a 2009 our study and the 2011 results from the KCDC10,14). This discrepancy is likely because the vaccination rates for rotavirus vary according to year and region13,14,15). Furthermore, age-specific vaccination schedules may affect the prevalence of different viral infections. Han et al. studied rotaviral and noroviral infections among children with gastroenteritis, and reported that these viruses were most commonly detected between the ages of 6 months and 2 years22,25,26). Similarly, we found that viral gastroenteritis was most prevalent between the ages of 6 months and 2 years. However, unlike noroviral infection (which occurred evenly among all age groups), rotaviral infection was most common among children who were <6 months old. This is likely because these infections developed before completion of the scheduled rotavirus vaccination. After 6 months, the prevalence of rotavirus tended to decrease for patients who were 2.4 years old. The increasing vaccination rates between 2011 and 2013 may also partially explain the lower prevalence of rotavirus among children aged less than 4 years13,14,15).
Previous studies have reported that norovirus and rotavirus exhibited similar peak prevalence6,27), but we found that rotavirus and norovirus exhibited peak prevalence at different times in this study. Norovirus reached its peak prevalence during December-February, while rotavirus was prevalent during February-March)28). Although the prevalence of adenovirus was lower than those of norovirus or rotavirus, it mainly occurred during June-August, and no cases were observed during December-January. Thus, adenovirus may be a summer-specific virus.
In children with a rotaviral infection, decreased urine output and weight loss (which indicate dehydration) were common, and fever was also common. This may be because the peak prevalence of rotavirus infection occurred among patients who were <6 months old, and these patients might have exhibited more severe dehydration symptoms, despite the same amount of vomiting and diarrhea. Several studies reported that patients with mixed infections presented with more severe clinical symptoms, which may be related to the synergistic action of multiple pathogens in exacerbating the duration and severity of the symptoms9,29,30). Although our study results revealed that 2.9% of patients had coinfections and a relatively high incidence of fever, the other clinical symptoms were similar to those of patients with a single infection.
The limitations of our study are its single center setting and short duration. Also, it has methodological problems due to the retrospective approach. We could not obtain the information about the rotavirus vaccination history from patients. Thus the direct causality between vaccination and rotavirus enteritis occurrence can not be concluded.
In conclusion, the most cause of acute pediatric viral gastroenteritis was norovirus, and its prevalence so high in witer season in our patient group. Therefore, it is important to prevent noroviral infections through the management of food and personal hygiene during the winter, and also to prevent adenoviral infections in water parks during the summer. Nevertheless, the prevalence of rotavirus has been decreased among children since 200831,32,33), it remains a major cause of diarrhea, which causes severe dehydration in children. Thus, it is critical that education regarding personal hygiene be implemented among daycare facilities that care for children who are <6 months old. Furthermore, a national program should be introduced to increase the vaccination rate for rotavirus, which is currently 30%-50%13). Moreover, continuous monitoring should be performed to detect new viral genotypes, which can indicate immune evasion and facilitate the development of additional vaccines. As norovirus is showing a trend of constant increase, it is also necessary to prevent infection within hospitals, and to prevent group outbreaks during the prevalent period, through active management of infection. Furthermore, there is a need for development of vaccines through viral genotype surveillance.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


