Article Contents
| Korean J Pediatr > Volume 57(12); 2014 |
|
Abstract
Hemorrhagic cystitis is a common stem cell transplantation-related complication. The incidence of early-onset hemorrhagic cystitis, which is related to the pretransplant conditioning regimen, has decreased with the concomitant use of mesna and hyperhydration. However, late-onset hemorrhagic cystitis, which is usually caused by the BK virus, continues to develop. Although the BK virus is the most common pathogenic microorganism of poststem cell transplantation late-onset hemorrhagic cystitis, pediatricians outside the hemato-oncology and nephrology specialties tend to be unfamiliar with hemorrhagic cystitis and the BK virus. Moreover, no standard guidelines for the early diagnosis and treatment of BK virus-associated hemorrhagic cystitis after stem cell transplantation have been established. Here, we briefly introduce poststem cell transplantation BK virus-associated hemorrhagic cystitis.
Hemorrhagic cystitis (HC) occurs in 9%-31% of all stem cell transplantation (SCT) recipients1,2,3,4,5,6). This complication is associated with increased morbidity and mortality, and particularly with urinary tract obstruction, renal dysfunction and renal failure, longer hospital stay, and increased hospital costs2,6,7,8,9,10). In the early period after SCT, chemotherapeutic agents such as cyclophosphamide and busulphan as well as irradiation administered during the pretransplant conditioning period can directly damage the bladder urothelium11,12,13,14,15). However, several complicating factors such as viral infection and acute graft-versus-host disease (GvHD) may cause HC in the late period following SCT3,11,14). In particular, a relationship between BK virus (BKV) infection and late-onset HC after SCT has been described1,3,16,17,18,19). Few reports have examined the impact of BKV infection on HC after SCT in Korea, where pediatric SCT has been performed since 198320,21).
This review will introduce the pathogenesis, clinical features, diagnosis, and treatment of BKV-associated HC after SCT.
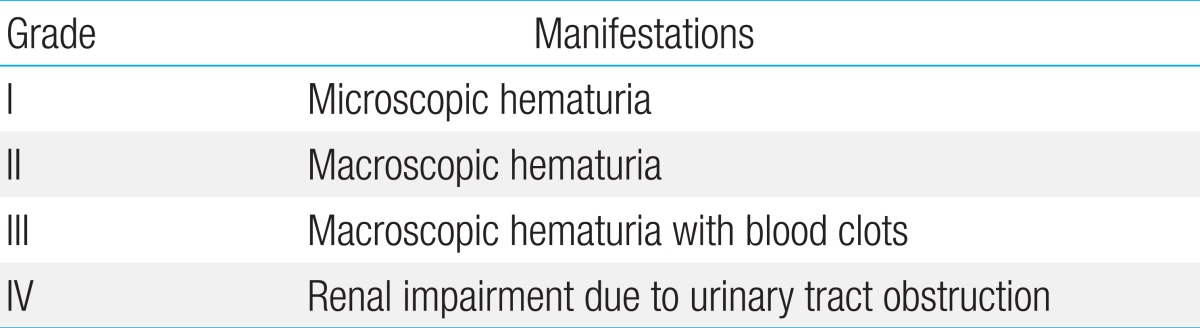
HC is defined as the development of microscopic or gross hematuria accompanied by lower urinary tract symptoms such as dysuria, frequent urination, urgency, and suprapubic pain. Other causes of bleeding such as bleeding tendency, bacterial or fungal infection, urinary tract mass, and vaginal bleeding should be excluded14,22). HC is categorized into four grades based on the severity of hematuria and its effect on the upper urinary tract (Table 1)14).
While a variety of time frames ranging from 48 hours to two weeks have been proposed1,12,19,23,24), post-SCT HC is generally divided into early-onset or pre-engraftment HC and late-onset or postengraftment HC. Early-onset HC is caused by chemotherapeutic agents including cyclophosphamide, ifosfamide, busulphan, and etoposide. It can also be caused by the irradiation administered to the pelvic area during the pretransplant conditioning period and by sustained thrombocytopenia prior to engraftment11,12,14,25). Sustained thrombocytopenia and coagulopathy may also cause HC in the late period after SCT. However, infections with viruses such as BKV, JC virus, cytomegalovirus (CMV), and adenovirus have been reported as major causes of late-onset HC after SCT1,11,17,18,19,23). Urinary BKV is detected in 35%-100% of SCT recipients with HC1,3,17), compared to ranges of 10%-15%9,26,27), 4%-26%9,23,27), and 4%-7%3,26) for adenovirus, CMV, and JC virus, respectively. Thus, BKV is the most important pathogenic microorganism of late-onset HC after SCT.
BKV is a member of the Polyomaviridae family and is a nonenveloped double-stranded DNA virus. First detected in 1970 in a postkidney transplant patient suffering from nephropathy, the virus was named "BK virus" after the patient's initials28). While BKV infects humans during childhood, a latent infection is maintained in the urinary tract16,29). Anti-BKV antibodies are present in approximately 80% of the general population and 91% of children aged 5-9 years30,31), and urinary BKV excretion is detected in 7%-14% of all immune-competent hosts30,32). Latent BKV is reactivated under immunosuppressed conditions, and BK viruria and viremia are detected in 53%-71%10,16,33) and 17%-51%10,34) of SCT recipients, respectively, regardless of HC status.
Late-onset HC after SCT is believed to develop in three phases14,16,33,35). The first is direct bladder mucosal damage caused by chemotherapeutic agents and irradiation received during pretransplant conditioning. During the second phase, BKV replication is activated in conjunction with the activated regeneration of damaged urothelial cells under the immunosuppressed status immediately following SCT. The third and final phase comprises an excessive inflammatory response of the reconstituted host immunity against activated BKV.
Although BK viruria were detected in 47%-52% of all SCT recipients1,26,33), only 38%-44% of them exhibited HC1,26). Moreover, 9%-50% of SCT recipients with HC did not exhibit BK viruria2,6,26,27,33). These findings indicate that factors other than BKV reactivation may contribute to the development of late-onset HC. Old age, acute GvHD, receiving stem cells from an unrelated donor, myeloablative conditioning, and allogeneic transplantation have all been suggested as possible contributing factors1,3,4,5,7,9,19,23,24,36,37).
BKV infection after kidney transplantation mainly manifests as BKV-associated nephropathy while BKV infection after SCT mainly manifests as HC25,35). In various studies, BKV-associated HC was found to occur 25-57 days (median values) after SCT, with symptoms lasting for 10-38 days (median values)3,4,8,16,17,20,34,38). In terms of severity, HC of grades I, II, III, and IV occurred in 0%-30%, 16%-57%, 36%-67% and 0%-12% of cases, respectively3,4,8,20,38). Conservative care tends to be effective in patients with lower grades of HC, while those with higher grades may suffer from renal complications and even death.
BKV-associated HC is diagnosed when an SCT recipient with grade II or higher HC complains of lower urinary tract symptoms such as dysuria, urinary frequency, urgency, and suprapubic pain and shows laboratory evidence of BKV replication39). Ultrasonography can be useful in the evaluation of blood clot formation and the severity of upper urinary tract complications22). Polymerase chain reaction (PCR)-mediated detection of BKV DNA is the modality of choice for diagnosing BK viruria11). BK viruria tend to precede clinical symptoms of HC, and therefore, PCR for urinary BKV can be useful in the early diagnosis of BKV-associated HC1,17,19,33). Previous studies reported that 106 copies/mL or 9×106 copies/mL of urinary BKV DNA was an amount significantly associated with the development of BKV-associated HC1,17). However, PCR for blood BKV DNA may be an even more valuable predictor of the development of BKV-associated HC since compared with BK viruria, BK viremia has been shown to be more significantly related to the severity of HC and the development of renal complications2,6,8,10). In addition, various cutoff values of blood BKV DNA titers, including 103 and 104 copies/mL, have been reported to predict the development of BKV-associated HC2,17,34).
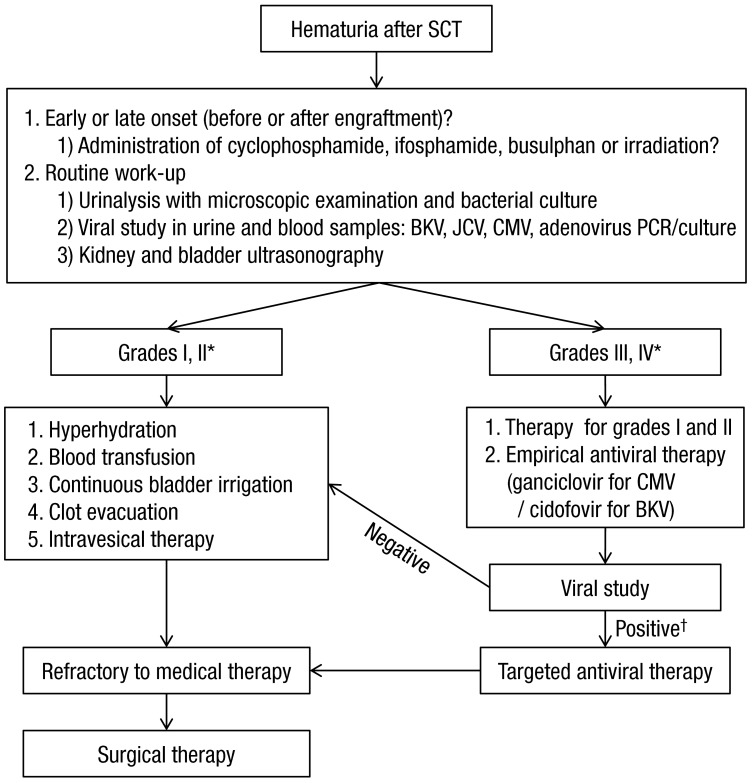
As mentioned above, late-onset HC after SCT can also be caused by other viruses such as adenovirus, CMV, JC virus, and human herpes virus 64,7,26,40). Therefore, blood PCR analyses, urinary PCR analyses, and urine cultures for other viruses should also be considered in SCT recipients with late-onset HC (Fig. 1).
Many therapeutic modalities have been applied in HC; however, standard therapeutic guidelines have yet to be established22,35). In addition, evaluating the efficacy of these diverse modalities in clinical trials is complicated by their frequent concurrent administration35). Treatments can be selected based on the patient's general condition and HC grade, and most patients recover with conservative care11). Cases that do not respond to conservative care should receive intravesical therapy or systemic therapy (Table 2). Finally, surgical therapy can be considered if hemorrhage and urinary tract complications do not improve with medical therapy. A proposed diagnostic and therapeutic algorithm for SCT recipients with HC is shown inFig. 1.
It is necessary to reduce or stop immunosuppressive therapies in patients with BKV-associated HC35). However, this cannot be achieved in most patients due to the possible aggravation of GvHD. Therefore, analgesic therapy and intensive intensive intravenous hydration (3 L/m2/day) with forced diuretics are administered as an initial treatment11,22). In addition, a platelet count >50,000/µL and hematocrit >25% should be maintained via blood transfusion11,22,35). If HC symptoms do not respond to these therapies or blood clots form in the bladder, continuous bladder irrigation with normal saline through a three-way urethral catheter should be performed22).
If hemorrhage does not improve or a urinary tract obstruction develops after 3 to 4 days of conservative care, cystoscopy for the complete evacuation of blood clots should be performed22).
If hemorrhage lasts for 7 to 10 days even after applying the aforementioned therapies, the intravesical instillation of topical agents should be considered22). Formalin, alum, prostaglandins, fibrin glue, and hyaluronic acid have all been applied intravesically as topical agents for HC7,8,9,23,41,42), but their efficacies have yet to be investigated in controlled studies. The use of formalin in children should be restricted due to the potential for severe complications including suprapubic pain, bladder scarring, and subsequent fibrosis and contracture22,43). While alum does not cause these local complications, the systemic absorption of aluminum may cause encephalopathy, seizures, and acidosis22,44,45).
Cidofovir is a cytidine nucleoside analog and an effective antiviral agent. Cidofovir has been shown to inhibit intracellular BKV replication in vitro46,47). In addition, multiple studies have demonstrated the clinical and microbiological effects of intravenous cidofovir on BKV-associated HC2,26,38,48,49,50). In our hospital, all 11 pediatric SCT recipients who received intravenous cidofovir therapy showed a clinical response, and 10 of the recipients showed a microbiological response20). Importantly, no severe side effects of cidofovir were observed20). Nevertheless, the therapeutic efficacy of cidofovir has not been comprehensively confirmed through controlled studies, and the most appropriate dosing regimen (1 mg/kg vs. 5 mg/kg) is still unclear. In addition, the nephrotoxicity of cidofovir should be considered in SCT recipients who receive several nephrotoxic drugs concomitantly.
Leflunomide, which is used in the treatment of rheumatoid arthritis, suppresses immune responses by inducing cytostasis, particularly in activated lymphocytes51,52). Since BKV relies on host factors for its replication, leflunomide is generally assumed to inhibit BKV replication by inhibiting DNA replication in BKV-infected cells51). The clinical and microbiological effects of leflunomide on BKV-associated HC in adult SCT recipients have been reported53). However, reports of its efficacy in children have been limited to kidney transplantation recipients54).
Although the quinolones are well-known as antibacterial drugs, they are also believed to inhibit intracellular BKV replication by inhibiting topoisomerase activity in BKV-infected mammalian cells55,56). The prophylactic and therapeutic effects of ciprofloxacin on BKV-associated HC in adult SCT recipients have been reported36,46). Quinolones are contraindicated in children younger than 18 years, and therefore, the effects of quinolones have not been studied in pediatric SCT recipients.
Estrogen has shown therapeutic efficacy against HC in children; it is believed that estrogen exerts these effects by stabilizing the microvasculature22,27). However, the evidence base consists of only a case report and a case series, neither of which was a controlled study. Moreover, other investigators have reported that estrogen is ineffective against HC7).
High-pressure oxygen generates a high oxygen gradient between the damaged urothelium and the surrounding healthy tissues. This oxygen gradient promotes macrophage invasion into the damaged tissues and stimulates angiogenesis and tissue healing via the secretion of cytokines by macrophages57). Therapeutic effects have been reported in adults and children with HC after SCT8,38,57), but only in case series and case reports. Moreover, this treatment requires specialized equipment for supplying high-pressure oxygen.
If HC is refractory to medical therapies, it may be treated with surgical interventions. Supravesical urinary diversion using a bilateral nephrostomy has shown efficacy in children with HC unresponsive to conservative care and intravesical therapy43). Supravesical urinary diversion prevents urokinase, which is secreted from renal cells, from reaching the bladder wall, thereby promoting bladder hemostasis43). Unfortunately, a life-threatening hemorrhage may require the selective embolization of vesical arteries or internal iliac arteries, and even cystectomies58,59). The proper indications and therapeutic efficacies of these surgical therapies have not yet been defined. Thus, future studies of surgical therapies for HC are required.
Although pediatric SCTs have been performed for approximately 30 years, only a few studies of HC, an SCT-related complication, have been reported in Korea. It is possible to predict the development of BKV-associated HC after SCT using urinary and blood BKV DNA titers. However, no standard guidelines for prophylactic or preemptive therapies have been established. A standard treatment for BKV-associated HC has also not been established. Therefore, various therapies are administered to patients based on the attending physician's decision. More studies aimed at establishing appropriate diagnostic and therapeutic guidelines for BKV-associated HC are necessary. Such efforts should be helpful in reducing SCT-related complications and mortality in SCT recipients.
References
1. Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J Clin Microbiol 2004;42:5394–5396.



2. Haines HL, Laskin BL, Goebel J, Davies SM, Yin HJ, Lawrence J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1512–1519.


3. Kloos RQ, Boelens JJ, de Jong TP, Versluys B, Bierings M. Hemorrhagic cystitis in a cohort of pediatric transplantations: incidence, treatment, outcome, and risk factors. Biol Blood Marrow Transplant 2013;19:1263–1266.


4. Laskin BL, Denburg M, Furth S, Diorio D, Goebel J, Davies SM, et al. BK viremia precedes hemorrhagic cystitis in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1175–1182.



5. Lee GW, Lee JH, Choi SJ, Kim S, Seol M, Kim WK, et al. Hemorrhagic cystitis following allogeneic hematopoietic cell transplantation. J Korean Med Sci 2003;18:191–195.



6. Oshrine B, Bunin N, Li Y, Furth S, Laskin BL. Kidney and bladder outcomes in children with hemorrhagic cystitis and BK virus infection after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1702–1707.



7. Cheuk DK, Lee TL, Chiang AK, Ha SY, Lau YL, Chan GC. Risk factors and treatment of hemorrhagic cystitis in children who underwent hematopoietic stem cell transplantation. Transpl Int 2007;20:73–81.


8. Gilis L, Morisset S, Billaud G, Ducastelle-Lepretre S, Labussiere-Wallet H, Nicolini FE, et al. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2014;49:664–670.


9. Hale GA, Rochester RJ, Heslop HE, Krance RA, Gingrich JR, Benaim E, et al. Hemorrhagic cystitis after allogeneic bone marrow transplantation in children: clinical characteristics and outcome. Biol Blood Marrow Transplant 2003;9:698–705.


10. O'Donnell PH, Swanson K, Josephson MA, Artz AS, Parsad SD, Ramaprasad C, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant 2009;15:1038–1048.e1.



11. Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant 2008;41:11–18.


12. Tsuboi K, Kishi K, Ohmachi K, Yasuda Y, Shimizu T, Inoue H, et al. Multivariate analysis of risk factors for hemorrhagic cystitis after hematopoietic stem cell transplantation. Bone Marrow Transplant 2003;32:903–907.


13. Brugieres L, Hartmann O, Travagli JP, Benhamou E, Pico JL, Valteau D, et al. Hemorrhagic cystitis following high-dose chemotherapy and bone marrow transplantation in children with malignancies: incidence, clinical course, and outcome. J Clin Oncol 1989;7:194–199.


14. Leung AY, Yuen KY, Kwong YL. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant 2005;36:929–937.


15. Seber A, Shu XO, Defor T, Sencer S, Ramsay N. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant 1999;23:35–40.


16. Bedi A, Miller CB, Hanson JL, Goodman S, Ambinder RF, Charache P, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol 1995;13:1103–1109.


17. Cesaro S, Facchin C, Tridello G, Messina C, Calore E, Biasolo MA, et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:363–370.


18. Erard V, Storer B, Corey L, Nollkamper J, Huang ML, Limaye A, et al. BK virus infection in hematopoietic stem cell transplant recipients: frequency, risk factors, and association with postengraftment hemorrhagic cystitis. Clin Infect Dis 2004;39:1861–1865.


19. Giraud G, Bogdanovic G, Priftakis P, Remberger M, Svahn BM, Barkholt L, et al. The incidence of hemorrhagic cystitis and BKviruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica 2006;91:401–404.

20. Kwon HJ, Kang JH, Lee JW, Chung NG, Kim HK, Cho B. Treatment of BK virus-associated hemorrhagic cystitis in pediatric hematopoietic stem cell transplant recipients with cidofovir: a single-center experience. Transpl Infect Dis 2013;15:569–574.


21. Han SH, Noh OK, Lee SW, Park SJ, Jung HJ, Park JE. Polyomavirus activation in pediatric patients with hemorrhagic cystitis following hematopoietic stem cell transplantation. Clin Pediatr Hematol Oncol 2012;19:92–99.
23. Cesaro S, Brugiolo A, Faraci M, Uderzo C, Rondelli R, Favre C, et al. Incidence and treatment of hemorrhagic cystitis in children given hematopoietic stem cell transplantation: a survey from the Italian association of pediatric hematology oncology-bone marrow transplantation group. Bone Marrow Transplant 2003;32:925–931.


24. Kondo M, Kojima S, Kato K, Matsuyama T. Late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation in children. Bone Marrow Transplant 1998;22:995–998.


25. Binet I, Nickeleit V, Hirsch HH. Polyomavirus infections in transplant recipients. Curr Opin Organ Transplant 2000;5:210–216.

26. Gorczynska E, Turkiewicz D, Rybka K, Toporski J, Kalwak K, Dyla A, et al. Incidence, clinical outcome, and management of virusinduced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2005;11:797–804.


27. Heath JA, Mishra S, Mitchell S, Waters KD, Tiedemann K. Estrogen as treatment of hemorrhagic cystitis in children and adolescents undergoing bone marrow transplantation. Bone Marrow Transplant 2006;37:523–526.


28. Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1971;1:1253–1257.

29. Heritage J, Chesters PM, McCance DJ. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J Med Virol 1981;8:143–150.


30. Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 2009;199:837–846.


31. Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 2003;71:115–123.


32. Polo C, Perez JL, Mielnichuck A, Fedele CG, Niubo J, Tenorio A. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin Microbiol Infect 2004;10:640–644.


33. Azzi A, Cesaro S, Laszlo D, Zakrzewska K, Ciappi S, De Santis R, et al. Human polyomavirus BK (BKV) load and haemorrhagic cystitis in bone marrow transplantation patients. J Clin Virol 1999;14:79–86.


34. Erard V, Kim HW, Corey L, Limaye A, Huang ML, Myerson D, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood 2005;106:1130–1132.



35. Rinaldo CH, Tylden GD, Sharma BN. The human polyomavirus BK (BKPyV): virological background and clinical implications. APMIS 2013;121:728–745.


36. Miller AN, Glode A, Hogan KR, Schaub C, Kramer C, Stuart RK, et al. Efficacy and safety of ciprofloxacin for prophylaxis of polyomavirus BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant 2011;17:1176–1181.


37. Rorije NM, Shea MM, Satyanarayana G, Hammond SP, Ho VT, Baden LR, et al. BK virus disease after allogeneic stem cell transplantation: a cohort analysis. Biol Blood Marrow Transplant 2014;20:564–570.


38. Megged O, Stein J, Ben-Meir D, Shulman LM, Yaniv I, Shalit I, et al. BK-virus-associated hemorrhagic cystitis in children after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol 2011;33:190–193.


39. Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009;15:1143–1238.



40. Kim YJ, Kim DW, Lee DG, Park ST, Park YH, Min CK, et al. Human herpesvirus-6 as a possible cause of encephalitis and hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Leukemia 2002;16:958–959.


41. Tirindelli MC, Flammia G, Sergi F, Cerretti R, Cudillo L, Picardi A, et al. Fibrin glue for refractory hemorrhagic cystitis after unrelated marrow, cord blood, and haploidentical hematopoietic stem cell transplantation. Transfusion 2009;49:170–175.


42. Miodosky M, Abdul-Hai A, Tsirigotis P, Or R, Bitan M, Resnick IB, et al. Treatment of post-hematopoietic stem cell transplantation hemorrhagic cystitis with intravesicular sodium hyaluronate. Bone Marrow Transplant 2006;38:507–511.


43. Lukasewycz SJ, Smith AR, Rambachan A, MacMillan ML, Lewis JM, Shukla AR. Intractable hemorrhagic cystitis after hematopoietic stem cell transplantation: is there a role for early urinary diversion in children? J Urol 2012;188:242–246.


44. Bogris SL, Johal NS, Hussein I, Duffy PG, Mushtaq I. Is it safe to use aluminum in the treatment of pediatric hemorrhagic cystitis? A case discussion of aluminum intoxication and review of the literature. J Pediatr Hematol Oncol 2009;31:285–288.


45. Kanwar VS, Jenkins JJ 3rd, Mandrell BN, Furman WL. Aluminum toxicity following intravesical alum irrigation for hemorrhagic cystitis. Med Pediatr Oncol 1996;27:64–67.


46. Leung AY, Chan MT, Yuen KY, Cheng VC, Chan KH, Wong CL, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2005;40:528–537.


47. Bernhoff E, Gutteberg TJ, Sandvik K, Hirsch HH, Rinaldo CH. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am J Transplant 2008;8:1413–1422.


48. Cesaro S, Hirsch HH, Faraci M, Owoc-Lempach J, Beltrame A, Tendas A, et al. Cidofovir for BK virus-associated hemorrhagic cystitis: a retrospective study. Clin Infect Dis 2009;49:233–240.


49. Cesaro S, Pillon M, Tridello G, Aljurf M, Martino R, Schroyens W, et al. Relationship between clinical and BK virological response in patients with late hemorrhagic cystitis treated with cidofovir: a retrospective study from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2013;48:809–813.


50. Faraci M, Cuzzubbo D, Lanino E, Di Marco E, Cirillo C, Dallorso S, et al. Low dosage cidofovir without probenecid as treatment for BK virus hamorrhagic cystitis after hemopoietic stem cell transplant. Pediatr Infect Dis J 2009;28:55–57.


51. Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol 2010;84:2150–2156.


52. Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med 1999;159:2542–2550.


53. Chen XC, Liu T, Li JJ, He C, Meng WT, Huang R. Efficacy and safety of leflunomide for the treatment of BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Acta Haematol 2013;130:52–56.


54. Araya CE, Garin EH, Neiberger RE, Dharnidharka VR. Leflunomide therapy for BK virus allograft nephropathy in pediatric and young adult kidney transplant recipients. Pediatr Transplant 2010;14:145–150.


55. Portolani M, Pietrosemoli P, Cermelli C, Mannini-Palenzona A, Grossi MP, Paolini L, et al. Suppression of BK virus replication and cytopathic effect by inhibitors of prokaryotic DNA gyrase. Antiviral Res 1988;9:205–218.


56. Ferrazzi E, Peracchi M, Biasolo MA, Faggionato O, Stefanelli S, Palu G. Antiviral activity of gyrase inhibitors norfloxacin, coumermycin A1 and nalidixic acid. Biochem Pharmacol 1988;37:1885–1886.


57. Zama D, Masetti R, Vendemini F, Di Donato F, Morelli A, Prete A, et al. Clinical effectiveness of early treatment with hyperbaric oxygen therapy for severe late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation in pediatric patients. Pediatr Transplant 2013;17:86–91.


Fig. 1
Diagnostic and therapeutic algorithm for stem cell transplant recipients with hemorrhagic cystitis. SCT, stem cell transplantation; BKV, BK virus; JCV, JC virus; CMV, cytomegalovirus; PCR, polymerase chain reaction. *SeeTable 1. †For BKV: urinary BKV DNA titer>107 copies/mL and serum BKV DNA titer>104 copies/mL.




 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


