< Previous Next >
Article Contents
| Clin Exp Pediatr > Volume 57(3); 2014 |
|
Abstract
The use of glucocorticoids (GCs) in the perinatal period is suspected of being associated with adverse effects on long-term neurodevelopmental outcomes for preterm infants. Repeated administration of antenatal GCs to mothers at risk of preterm birth may adversely affect fetal growth and head circumference. Fetal exposure to excess GCs during critical periods of brain development may profoundly modify the limbic system (primarily the hippocampus), resulting in long-term effects on cognition, behavior, memory, co-ordination of the autonomic nervous system, and regulation of the endocrine system later in adult life. Postnatal GC treatment for chronic lung disease in premature infants, particularly involving the use of dexamethasone, has been shown to induce neurodevelopmental impairment and increases the risk of cerebral palsy. In contrast to studies involving postnatal dexamethasone, long-term follow-up studies for hydrocortisone therapy have not revealed adverse effects on neurodevelopmental outcomes. In experimental studies on animals, GCs has been shown to impair neurogenesis, and induce neuronal apoptosis in the immature brains of newborn animals. A recent study has demonstrated that dexamethasone-induced hypomyelination may result from the apoptotic degeneration of oligodendrocyte progenitors in the immature brain. Thus, based on clinical and experimental studies, there is enough evidence to advice caution regarding the use of GCs in the perinatal period; and moreover, the potential long-term effects of GCs on brain development need to be determined.
Although glucocorticoids have been widely used in the perinatal period, their use is suspected of being associated with adverse effects on fetal growth and long-term neurodevelopmental outcomes in preterm infants1,2,3,4,5). Since the trials conducted by Liggins and Howie in 19726), many observational and controlled studies have reported that antenatal administration of glucocorticoids (GCs) to mothers at risk of preterm birth decreases the severity of respiratory distress syndrome and improves the survival of preterm infants6,7,8,9,10). However, repeated courses of antenatal GCs may be associated with reduced fetal brain growth and long-term neurodevelopmental impairment10,11,12,13,14,15,16,17). Several studies of systematic reviews and meta-analyses have shown that postnatal use of GCs to prevent or treat chronic lung disease (CLD) in preterm infants may facilitate extubation and decrease the incidence of bronchopulmonary dysplasia (BPD), but have found that it increases the risk of neurodevelopmental impairment and cerebral palsy (CP), particularly in infants treated with dexamethasone within the first week of life2,3,4,5,18,19,20,21,22,23,24). In addition, fetal exposure to excessive GCs may induce a significant change in the hypothalamic-pituitary adrenal (HPA) axis25,26,27,28,29). Studies using magnetic resonance imaging (MRI) have demonstrated a significant reduction in brain tissue volumes in infants treated with GCs in the perinatal period24,30,31,32,33,34). The purpose of this review was to evaluate clinical and experimental evidence for the adverse impact of GCs on the developing brain.
A relatively brief course of antenatal GCs administration (dexamethasone or betamethasone) improves survival and appears to protect against brain damage2,10,35,36,37). In clinical trials and observational studies, antenatal administration of GCs has been associated with a decreased risk of intraventricular hemorrhage and CP in preterm infants, and confers protection against periventricular leukomalacia35,36,37). The protective effect on the white matter appears to be greater among infants who show evidence of a fetal inflammatory response, such as chorioamnionitis36,37). In one retrospective study, antenatal exposure to betamethasone, but not dexamethasone, was associated with the decreased risk of cystic periventricular leukomalacia in premature infants of 24 to 31 weeks gestation37). With regard to neurodevelopmental outcomes, a single course of antenatal GCs is generally thought to be beneficial14,38,39,40,41,42,43). However, the use of repeated courses of antenatal GCs has been more controversial, despite some evidence for neonatal benefit if the mother remains at risk of preterm labor29). Several nonrandomized studies concerning repeated courses of antenatal GCs have reported adverse effects on fetal growth and head circumference, suppression of the fetal HPA axis, and abnormal neurodevelopment13,27,28). Repeated betamethasone injections, given to the mother at weekly intervals, have resulted in improvements in postnatal lung function, but also a reduction in birth weights42). In studies using volumetric MRI, repeated antenatal GC treatments have been associated with a decreases in brain surface area, in the whole cortex convolution index, and in a measure of cortical surface complexity in preterm infants43). A study by the Australasian Collaborative Trial of Repeat Doses of Steroids reported that repeated administration of antenatal GCs to mothers at risk of preterm birth reduced neonatal morbidity, without changing either survival free of major neurosensory disability or body size at 2 years of ages, although z-scores for weight and head circumference were lower at birth in the repeated-dose group compared with the single-dose group16). One systematic review reported that repeated administration of betamethasone weekly or biweekly restricted intrauterine growth, which has raised concerns about long-term consequences on neurodevelopment and metabolism14).
Endogenous GCs are essential for many aspects of normal brain development. However, dexamethasone and betamethasone readily cross the placental barrier, potentially exposing the fetus to excess GCs29), which can suppress the HPA axis and endogenous cortisol production27,28,29,33). Overexposure of the fetus to corticosteroids by using synthetic GCs during certain stages of pregnancy can profoundly affect the development of the neuroendocrine system which may lead to life-long effects on endocrine, behavioral, emotional, and cognitive function. These life-long effects on neuroendocrine function are associated with increased risks of developing a wide range of metabolic, cardiovascular, and brain disorders in later life25,26).
In summary, a single course of antenatal GCs administered to mothers at risk of premature labor has major benefits for infants born before 34 weeks gestation6,7,8,9,10,35,36,37,38,39,40,41). However, there is limited information available on the long-term effects of antenatal GCs on neurodevelopment in humans. Repeated courses of antenatal CGs may be associated with reduced birth weight and fetal head growth. Fetal exposure to excess GCs can affect the development of the neuroendocrine system, potentially leading to life-long effects on endocrine, behavior, emotional, and cognitive function. More studies including long-term follow-up of exposed children are needed to confirm the efficacy and safety of antenatal GCs use, especially of repeated courses of treatment.
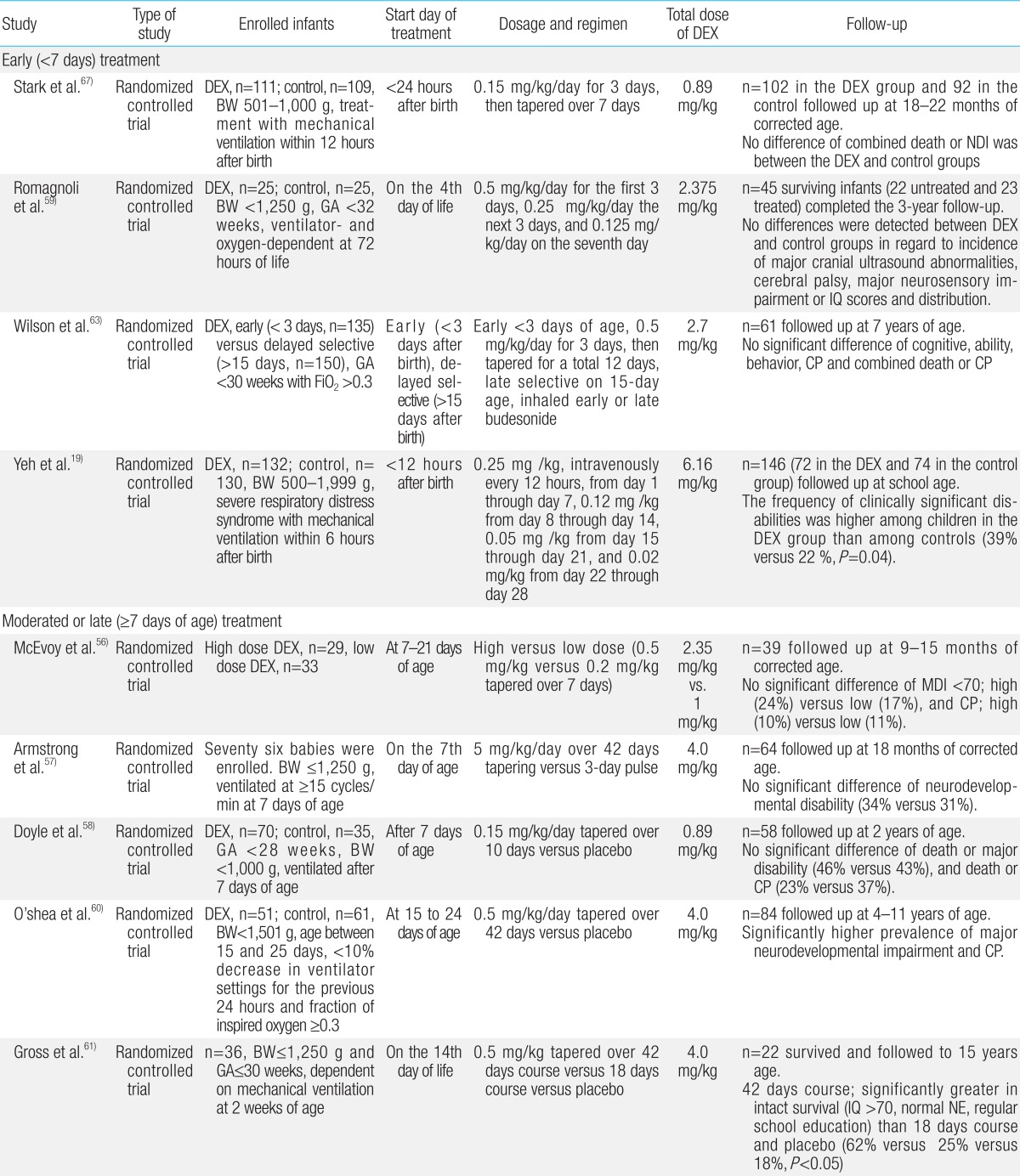
Since the first report in 1974, a number of studies have showed that use of postnatal GCs to treat or prevent CLD increases the risk of neurodevelopmental disability and CP in preterm infants19,20,21,22,23,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67). Among systemically administered postnatal GCs, dexamethasone has been extensively studied and has proven to be effective in the management of BPD because of its anti-inflammatory effects45). Dexamethasone is a potent, long-acting steroid with exclusively glucocorticoid effects. Its use has been studied during the early (<7 days), moderately early (7-14 days) and late/delayed (>14 days) postnatal periods at doses ranging from 0.1 mg/kg/day to 0.5 mg/kg/day, and durations ranging from 3 to 42 days2,4,5,21,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63). In recent systematic meta-analysis reviews, early postnatal treatment with dexamethasone within the first week of birth was shown to induce neurodevelopmental disability and increase the risk of CP, despite its short-term beneficial effects on the lung function21). In contrast, one extensive systematic review showed that treatment with dexamethasone after the first week of postnatal life may actually reduce mortality rates or the incidence of long-term neurodevelopmental disability although long-term follow-up data remains limited55). In addition, it has been suggested that high dosage (0.5 mg/kg/day) and long duration of treatment may be the factors responsible for delays in brain growth and poor neurodevelopmental outcomes64). Follow-up data reported by Wilson-Costello et al.65) in 2009 has suggested that every for every 1 mg/kg increase in the cumulative dose of dexamethasone, the risk of developing disabling CP increased by 40% at every gestational age. However, in a systematic review of placebo-controlled trials, Onland et al.66) reported that higher cumulative dexamethasone doses after the first week of life decreased the risk of BPD without increasing the risk of neurodevelopmental disability in ventilated preterm infants, and had no effect on neurodevelopmental outcomes after the third week of life. The severity of CLD can modify the effect of steroids on CP. One meta-analysis by Doyle et al.51) showed that postnatal treatment with GCs increased the likelihood of death or CP in infants whose risk of CLD was below 35%, but reduced the likelihood of death or CP if the risk of CLD exceeded 65%. The results of randomized controlled trials (RCTs) that have been performed to evaluate long-term neurodevelopmental outcomes from postnatal dexamethasone use are summarized in Table 1. The results vary. Some studies did not reveal adverse effects on neurodevelopmental outcomes at various ages. In particular, the results from two RCTs using lower doses of dexamethasone (0.15 mg/kg/day for 3 days, then tapering over 7 days) did not show a significant increase in CP and neurodevelopmental impairment when compared with placebo23,58).
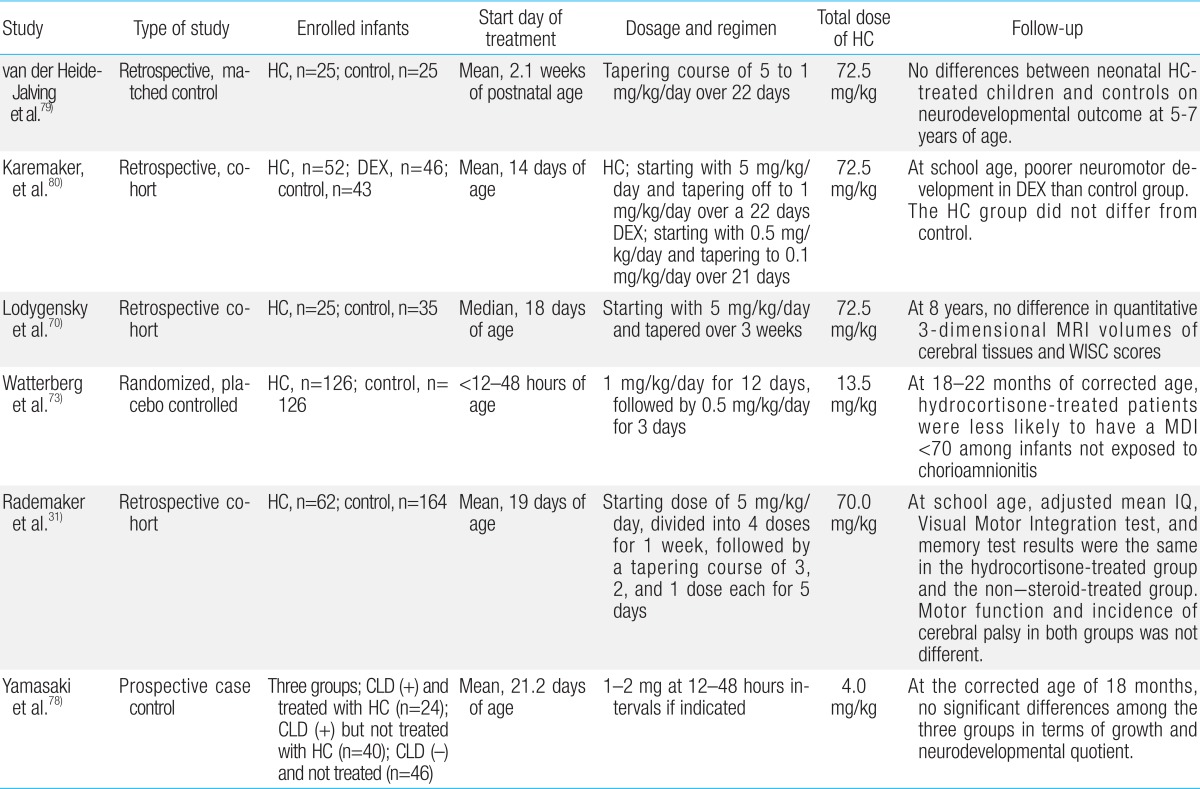
Hydrocortisone is the second most commonly used postnatal GC in premature infants. Extremely premature infants have developmental immaturity of the HPA axis during the first few weeks of life, predisposing them to relative adrenal insufficiency and inadequate anti-inflammatorty defenses against acute illness68). Consequently, early physiological replacement of cortisol may be needed in extremely premature infants68). Retrospective studies and RCTs of early hydrocortisone replacement for CLD within the first week of life have been performed on the basis of this relative adrenal insufficiency69,70,71,72,73,74,75,76,77,78,79,80). Hydrocortisone is also being used increasingly for the treatment or prevention of vasopressor-resistant hypotension in extremely premature infants68). In contrast to postnatal dexamethasone, the long-term follow-up studies for hydrocortisone therapy have not revealed adverse effects on neurodevelopmental outcomes79,80). In a retrospective study, van der Heide-Jalving et al.79) reported that no differences in neurodevelopment outcomes at 5-7 years of age were found between the hydrocortisone-treated group (n=25) and control subjects (n=25). Watterberg et al.73) evaluated a total of 252 infants from 291 survivors with birth weights of 500-999 g at 18 to 22 months corrected age. Low doses of hydrocortisone treatment (1 mg/kg/day for 12 days, followed by 0.5 mg/kg/day for 3 days) was not associated with increased rates of developmental delay and CP in surviving infants73). However, hydrocortisone significantly increased the risks of spontaneous gastro-intestinal perforation69). This complication was also observed with early dexamethasone treatment and may result from interactions with indomethacin/ibuprofen23,69). Low (1 mg/kg/day) or relatively high doses (3-6 mg/kg/day) of hydrocortisone treatment in neonates has had no discernible effect on brain lesions and total or regional brain volumes measured using MRI in short-term or long-term follow-up studies continued until school age31). In a recent meta-analysis of eight RCTs enrolling a total of 880 infants, postnatal treatment with low (1-2 mg/kg/day) or high dose (3-6 mg/kg/day) hydrocortisone has not shown any adverse effects on neurodevelopment22). This study has demonstrated that there is little evidence for a direct effect of hydrocortisone on the rates of BPD, mortality, or the combined outcome of BPD or mortality22). The results of long-term outcome studies of postnatal hydrocortisone use are summarized in Table 2.
There are several possible explanations for these differences between the effects of dexamethasone and hydrocortisone on brain growth and neurodevelopment45,81,82). Hydrocortisone has almost equal glucocorticoid and mineralocorticoid actions, and its half-life is only 8 hours. When compared to hydrocortisone, dexamethasone has exclusive glucocorticoids action, is 25-50 times more potent, and its half-life is 36-54 hours45). High-dose dexamethasone (0.5 mg/kg/day) is equivalent to at least a 15-20 mg/kg/day dose of hydrocortisone, far higher than the range of doses of hydrocortisone given in the studies discussed above (1-6 mg/kg/day). Even low-dose dexamthasone (0.1-0.15 mg/kg/day) may be equivalent to a 3-6 mg/kg/day dose of hydrocortisone, but has a longer half-life45,46,83). Therefore, dexamethasone may have a much higher relative potency than hydrocortisone, predisposing recipients to an increased risk of neurodevelopmental disability. Dissimilar effects of these two agents on the hippocampus have also been reported in animal studies. The hippocampus contains high densities of both mineralocorticoid and glucocorticoid receptors (GRs)81,82). Dexamethasone, which binds only to GRs, has been shown to induce neurodegeneration within the hippocampus84,85). In humans, neonatal treatment with dexamethasone, but not hydrocortisone, has been shown to alter hippocampal synaptic plasticity and associative memory formation in later life1). Dexamethasone exposure has also been linked to decreased hippocampal volume, but cohort studies of infants treated with hydrocortisone have not revealed a decrease in hippocampal volume31,33,34,70,71).
In summary, when considering postnatal GCs use, the least toxic GC should be given at the lowest effective dose for the shortest possible time75). Use of postnatal CGs should be limited to those who are expected to need prolonged ventilation, and have a higher risk of development of CLD2,4). Particularly, dexamethasone should not be given in the first week of life unless the treatment is life-saving, and after the second week of life, its use should be restricted to those infants whose ventilator requirements suggest that they are at high risk of developing severe CLD5). The follow-up studies tend to favor hydrocortisone as a safer alternative to dexamethasone. However, follow-up studies on postnatal dexamethasone treatment were placebo-controlled randomized studies, whereas most of hydrocortisone follow-up studies were retrospective cohort studies (Tables 1, 2). The use hydrocortisone as a safer alternative to dexamethasone requires more evidence to confirm that hydrocortisone is safer in term of long-term neurological outcomes.
Corticosteroids are essential for normal brain development. Two types of corticosteroid receptor: mineralocorticoid receptors (MRs) and GRs81). MRs are predominantly expressed in the limbic structures (primarily the hippocampus), whereas GRs are more diffusely distributed with highest levels in the limbic system, hypothalamic paraventricular nucleus, and the cerebral cortex. Endogenous GC (cortisol in humans, corticosterone in rats) binds to MRs with a higher affinity than to GRs1,81,82). Under basal conditions, MRs are predominantly occupied, while, in the stressed condition, in which the level of cortisol is increased, the occupation of GRs increases1,82). Within the developing brain, the limbic system is particularly sensitive to endogenous and exogenous GCs82). A study using guinea pigs has demonstrated that fetal exposure to exogenous GCs modified the expression of GR and MR mRNA in the hippocampus and dentate gyrus84). Synthetic GCs (dexamethasone and betamethasone) bind predominantly to the GRs. Therefore, the impact of exogenous synthetic GCs on brain development is likely mediated by modification of GR expression in the brain, and may be dependent on the level of expression of GRs at the time of exposure1,26).
Changes in the expression of GRs and MRs in the hippocampus can result in long-term modification of the HPA axis25,26). Fetal exposure to exogenous GCs during the critical periods of brain development may exert a profound influence on the limbic system (primarily the hippocampus), resulting in long-term changes in cognition, behavior, memory, co-ordination of the autonomic nervous system, and regulation of a number of endocrine system functions later in adult life26,28,29). Exposure to synthetic GCs in utero results in hyperactivity of the HPA axis in adults, which will have a long-term impact on health24,25,26,27,28,29). There is growing evidence that prenatal exposure to GCs may be linked to the premature onset of cardiovascular and metabolic diseases, such as hypertension and diabetes26,28,29).
Animal studies have shown that GCs, especially dexamethasone, impaired neurogenesis and induced neuronal apoptosis85,86). Repeated administration of dexamethasone to neonatal rats has resulted in dose-dependent decrease in the neurogenesis in the subventricular zone, subgranular zone and cortex, and also resulted in a decrease in the brain weight86). Exposure of the immature mouse brain to clinically relevant doses of GCs has been shown to cause apoptosis of neural progenitor cells in the external granular cell layer of the developing mouse cerebellum, and leads to permanent decreases in the number of cerebellar neurons87,88). Dexamethasone treatment in neonatal rats has led to significantly decreased brain weights and increased cleaved casepase-3 levels in the cortex, thalamus, hippocampus, cerebellum, dentate gyrus and subventricular zone89). These findings suggest that the decrease in brain weight and the accompanying neurodevelopmental impairment may be due to impaired neurogenesis and/or degeneration of neurons by GCs.
The involvement of corticosteroids in myelin biosynthetic pathways is well known89,90). Dexamethasone affects myelin basic protein (MBP) synthesis by modulating gene expression, or by acting on GRs91). Repeated administration of dexamethasone has been shown to adversely affect the myelination of the developing rat brain, and disturbs myelin synthesis in fetal sheep92). Several studies have suggested that GCs affect the maturation of oligodendrocytes (OLs) and impair the formation of myelin90,91,92,93). Other studies have reported that corticosteroids enhance the expression of genes related to the synthesis of MBP and exert trophic and protective effects in the nervous system93,94). A study using cultured cells from rat brain has shown that dexamethasone alters oligodendroglial differentiation and myelination depending on the stage: early in the myelination process, dexamethasone has a stimulatory effect, whereas at later stages, it causes marked inhibition95). In addition, administration of GCs during critical periods of brain development may impair neurogenesis and myelination86,87,88,89,90,91). However, the precise mechanisms behind the adverse effects of GCs on myelination in the developing brain are not well understood. A recent study has shown that administration of dexamethasone during critical periods of brain development induces degenerative morphological changes in OL progenitors, suggestive of apoptosis, and subsequently resulting in hypomyelination (Fig. 1)90). This finding suggests that dexamethasone-induced hypomyelination may be, at least partially, due to injury to OL progenitors during specific stages in the maturation of OLs, as well as inhibition of myelin formation90).
Although GCs have been widely used during the antenatal and postnatal period, many issues relating to neurodevelopment remain unclear. A body of clinical and experimental evidences suggests that exposure to exogenous GCs during critical periods of brain development may exert profound effects on subsequent brain development and the regulation of a number of neuroendocrine systems function in later adult life. Thus, based on these clinical and experimental studies, there is enough evidence to advice caution in the use of GCs in the perinatal period. And when considering postnatal GCs use, the least toxic GC should be given at the lowest effective dose for the shortest possible time. Further research is required determine the potential long-term effects and mechanisms of action of GCs on brain development.
References
1. Matthews SG. Antenatal glucocorticoids and the developing brain: mechanisms of action. Semin Neonatol 2001;6:309–317.


2. Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002;109:330–338.


4. Watterberg KL. American Academy of Pediatrics. Committee on Fetus and Newborn. Policy statement: postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126:800–808.


5. Yates HL, Newell SJ. Postnatal intravenous steroids and long-term neurological outcome: recommendations from meta-analyses. Arch Dis Child Fetal Neonatal Ed 2012;97:F299–F303.


6. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515–525.



7. Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. Br J Obstet Gynaecol 1989;96:401–410.


8. Chapman SJ, Hauth JC, Bottoms SF, Iams JD, Sibai B, Thom E, et al. Benefits of maternal corticosteroid therapy in infants weighing </=1000 grams at birth after preterm rupture of the amnion. Am J Obstet Gynecol 1999;180(3 Pt 1): 677–682.


9. Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Short and long-term effects of antenatal corticosteroids assessed in a cohort of 7,827 children born preterm. Acta Obstet Gynecol Scand 2009;88:933–938.


10. Malloy MH. Antenatal steroid use and neonatal outcome: United States 2007. J Perinatol 2012;32:722–727.


11. Battin M, Bevan C, Harding J. Growth in the neonatal period after repeat courses of antenatal corticosteroids: data from the ACTORDS randomised trial. Arch Dis Child Fetal Neonatal Ed 2012;97:F99–F105.


12. Peltoniemi OM, Kari MA, Hallman M. Repeated antenatal corticosteroid treatment: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2011;90:719–727.


13. Norberg H, Stalnacke J, Diaz Heijtz R, Smedler AC, Nyman M, Forssberg H, et al. Antenatal corticosteroids for preterm birth: dose-dependent reduction in birthweight, length and head circumference. Acta Paediatr 2011;100:364–369.


14. Peltoniemi OM, Kari MA, Lano A, Yliherva A, Puosi R, Lehtonen L, et al. Two-year follow-up of a randomised trial with repeated antenatal betamethasone. Arch Dis Child Fetal Neonatal Ed 2009;94:F402–F406.


15. Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med 2007;357:1190–1198.


16. Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med 2007;357:1179–1189.


17. Murphy KE, Willan AR, Hannah ME, Ohlsson A, Kelly EN, Matthews SG, et al. Effect of antenatal corticosteroids on fetal growth and gestational age at birth. Obstet Gynecol 2012;119:917–923.


18. Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics 1998;101:E7


19. Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 2004;350:1304–1313.


20. Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed 2000;83:F177–F181.



21. Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98:217–224.


22. Doyle LW, Ehrenkranz RA, Halliday HL. Postnatal hydrocortisone for preventing or treating bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98:111–117.


23. Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 2001;344:95–101.


24. Sizonenko SV, Borradori-Tolsa C, Bauthay DM, Lodygensky G, Lazeyras F, Huppi P. Impact of intrauterine growth restriction and glucocorticoids on brain development: insights using advanced magnetic resonance imaging. Mol Cell Endocrinol 2006;254-255:163–171.


25. Champagne DL, de Kloet ER, Joels M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Semin Fetal Neonatal Med 2009;14:136–142.


26. Tegethoff M, Pryce C, Meinlschmidt G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocr Rev 2009;30:753–789.


27. Li J, Wang ZN, Chen YP, Dong YP, Shuai HL, Xiao XM, et al. Late gestational maternal serum cortisol is inversely associated with fetal brain growth. Neurosci Biobehav Rev 2012;36:1085–1092.


28. Waffarn F, Davis EP. Effects of antenatal corticosteroids on the hypothalamic-pituitary-adrenocortical axis of the fetus and newborn: experimental findings and clinical considerations. Am J Obstet Gynecol 2012;207:446–454.



29. Reynolds RM. Antenatal glucocorticoid treatment for preterm birth: considerations for the developing foetus. Clin Endocrinol (Oxf) 2013;78:665–666.


30. Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics 2001;107:217–221.


31. Rademaker KJ, Uiterwaal CS, Groenendaal F, Venema MM, van Bel F, Beek FJ, et al. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. J Pediatr 2007;150:351–357.


32. Parikh NA, Lasky RE, Kennedy KA, Moya FR, Hochhauser L, Romo S, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics 2007;119:265–272.


33. Parikh NA, Kennedy KA, Lasky RE, McDavid GE, Tyson JE. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. J Pediatr 2013;162:685–690.


34. Kersbergen KJ, de Vries LS, van Kooij BJ, Isgum I, Rademaker KJ, van Bel F, et al. Hydrocortisone treatment for bronchopulmonary dysplasia and brain volumes in preterm infants. J Pediatr 2013;163:666–671.


35. Canterino JC, Verma U, Visintainer PF, Elimian A, Klein SA, Tejani N. Antenatal steroids and neonatal periventricular leukomalacia. Obstet Gynecol 2001;97:135–139.


36. Agarwal R, Chiswick ML, Rimmer S, Taylor GM, McNally RJ, Alston RD, et al. Antenatal steroids are associated with a reduction in the incidence of cerebral white matter lesions in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2002;86:F96–F101.



37. Baud O, Foix-L'Helias L, Kaminski M, Audibert F, Jarreau PH, Papiernik E, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med 1999;341:1190–1196.


38. Gaillard EA, Cooke RW, Shaw NJ. Improved survival and neurodevelopmental outcome after prolonged ventilation in preterm neonates who have received antenatal steroids and surfactant. Arch Dis Child Fetal Neonatal Ed 2001;84:F194–F196.



39. Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics 2005;116:635–643.


40. Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR, et al. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed 2005;90:F134–F140.



41. Chawla S, Bapat R, Pappas A, Bara R, Zidan M, Natarajan G. Neurodevelopmental outcome of extremely premature infants exposed to incomplete, no or complete antenatal steroids. J Matern Fetal Neonatal Med 2013;26:1542–1547.


42. Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med 1997;156:178–184.


43. Modi N, Lewis H, Al-Naqeeb N, Ajayi-Obe M, Dore CJ, Rutherford M. The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatr Res 2001;50:581–585.


44. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Paediatr Child Health 2002;7:20–46.



45. Gupta S, Prasanth K, Chen CM, Yeh TF. Postnatal corticosteroids for prevention and treatment of chronic lung disease in the preterm newborn. Int J Pediatr 2012;2012:315642

46. Fitzhardinge PM, Eisen A, Lejtenyi C, Metrakos K, Ramsay M. Sequelae of early steroid administration to the newborn infant. Pediatrics 1974;53:877–883.



47. Cummings JJ, D'Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med 1989;320:1505–1510.


48. Jones R, Wincott E, Elbourne D, Grant A. Controlled trial of dexamethasone in neonatal chronic lung disease: a 3-year follow-up. Pediatrics 1995;96(5 Pt 1): 897–906.

49. O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG 3rd, et al. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics 1999;104(1 Pt 1): 15–21.


50. Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr 2001;1:1



51. Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics 2005;115:655–661.


52. Vincer MJ, Allen AC, Joseph KS, Stinson DA, Scott H, Wood E. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics 2006;118:e1621–e1626.


53. LeFlore JL, Engle WD. Growth and neurodevelopment in extremely low-birth-weight neonates exposed to postnatal steroid therapy. Am J Perinatol 2011;28:635–642.


54. Crotty KC, Ahronovich MD, Baron IS, Baker R, Erickson K, Litman FR. Neuropsychological and behavioral effects of postnatal dexamethasone in extremely low birth weight preterm children at early school age. J Perinatol 2012;32:139–146.


55. Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98:289–296.


56. McEvoy C, Bowling S, Williamson K, McGaw P, Durand M. Randomized, double-blinded trial of low-dose dexamethasone: II. Functional residual capacity and pulmonary outcome in very low birth weight infants at risk for bronchopulmonary dysplasia. Pediatr Pulmonol 2004;38:55–63.


57. Armstrong DL, Penrice J, Bloomfield FH, Knight DB, Dezoete JA, Harding JE. Follow up of a randomised trial of two different courses of dexamethasone for preterm babies at risk of chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2002;86:F102–F107.



58. Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB. DART Study Investigators. Outcome at 2 years of age of infants from the DART study: a multicenter, international, randomized, controlled trial of low-dose dexamethasone. Pediatrics 2007;119:716–721.


59. Romagnoli C, Zecca E, Luciano R, Torrioli G, Tortorolo G. Controlled trial of early dexamethasone treatment for the prevention of chronic lung disease in preterm infants: a 3-year follow-up. Pediatrics 2002;109:e85


60. O'Shea TM, Washburn LK, Nixon PA, Goldstein DJ. Follow-up of a randomized, placebo-controlled trial of dexamethasone to decrease the duration of ventilator dependency in very low birth weight infants: neurodevelopmental outcomes at 4 to 11 years of age. Pediatrics 2007;120:594–602.


61. Gross SJ, Anbar RD, Mettelman BB. Follow-up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics 2005;115:681–687.


62. Jones RA. Collaborative Dexamethasone Trial Follow-up Group. Randomized, controlled trial of dexamethasone in neonatal chronic lung disease: 13- to 17-year follow-up study: I. Neurologic, psychological, and educational outcomes. Pediatrics 2005;116:370–378.


63. Wilson TT, Waters L, Patterson CC, McCusker CG, Rooney NM, Marlow N, et al. Neurodevelopmental and respiratory follow-up results at 7 years for children from the United Kingdom and Ireland enrolled in a randomized trial of early and late postnatal corticosteroid treatment, systemic and inhaled (the Open Study of Early Corticosteroid Treatment). Pediatrics 2006;117:2196–2205.


64. Onland W, De Jaegere AP, Offringa M, van Kaam AH. Effects of higher versus lower dexamethasone doses on pulmonary and neurodevelopmental sequelae in preterm infants at risk for chronic lung disease: a meta-analysis. Pediatrics 2008;122:92–101.


65. Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months' adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 2009;123:e430–e437.



66. Onland W, Offringa M, De Jaegere AP, van Kaam AH. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics 2009;123:367–377.


67. Stark AR, Carlo WA, Vohr BR, Papile LA, Saha S, Bauer CR, et al. Death or neurodevelopmental impairment at 18 to 22 months corrected age in a randomized trial of early dexamethasone to prevent death or chronic lung disease in extremely low birth weight infants. J Pediatr 2014;164:34–39.e2.


68. Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2004;89:F119–F126.



69. Peltoniemi O, Kari MA, Heinonen K, Saarela T, Nikolajev K, Andersson S, et al. Pretreatment cortisol values may predict responses to hydrocortisone administration for the prevention of bronchopulmonary dysplasia in high-risk infants. J Pediatr 2005;146:632–637.


70. Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 2005;116:1–7.


71. Benders MJ, Groenendaal F, van Bel F, Ha Vinh R, Dubois J, Lazeyras F, et al. Brain development of the preterm neonate after neonatal hydrocortisone treatment for chronic lung disease. Pediatr Res 2009;66:555–559.



72. Rademaker KJ, de Vries LS, Uiterwaal CS, Groenendaal F, Grobbee DE, van Bel F. Postnatal hydrocortisone treatment for chronic lung disease in the preterm newborn and long-term neurodevelopmental follow-up. Arch Dis Child Fetal Neonatal Ed 2008;93:F58–F63.


73. Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007;120:40–48.


74. Bonsante F, Latorre G, Iacobelli S, Forziati V, Laforgia N, Esposito L, et al. Early low-dose hydrocortisone in very preterm infants: a randomized, placebo-controlled trial. Neonatology 2007;91:217–221.


75. Rademaker KJ, de Vries WB. Long-term effects of neonatal hydrocortisone treatment for chronic lung disease on the developing brain and heart. Semin Fetal Neonatal Med 2009;14:171–177.


76. Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari MA, et al. Trial of early neonatal hydrocortisone: two-year follow-up. Neonatology 2009;95:240–247.


77. Needelman H, Hoskoppal A, Roberts H, Evans M, Bodensteiner JB. The effect of hydrocortisone on neurodevelopmental outcome in premature infants less than 29 weeks gestation. J Child Neurol 2010;25:448–452.


78. Yamasaki C, Uchiyama A, Nakanishi H, Masumoto K, Aoyagi H, Washio Y, et al. Hydrocortisone and long-term outcomes in very-low-birthweight infants. Pediatr Int 2012;54:465–470.


79. van der Heide-Jalving M, Kamphuis PJ, van der Laan MJ, Bakker JM, Wiegant VM, Heijnen CJ, et al. Short- and long-term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone? Acta Paediatr 2003;92:827–835.


80. Karemaker R, Heijnen CJ, Veen S, Baerts W, Samsom J, Visser GH, et al. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatr Res 2006;60:745–750.


81. Rashid S, Lewis GF. The mechanisms of differential glucocorticoid and mineralocorticoid action in the brain and peripheral tissues. Clin Biochem 2005;38:401–409.


82. De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998;19:269–301.


83. Inder TE, Benders M. Postnatal steroids in the preterm infant-the good, the ugly, and the unknown. J Pediatr 2013;162:667–670.


84. Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res 1999;846:253–259.


85. Haynes LE, Griffiths MR, Hyde RE, Barber DJ, Mitchell IJ. Dexamethasone induces limited apoptosis and extensive sublethal damage to specific subregions of the striatum and hippocampus: implications for mood disorders. Neuroscience 2001;104:57–69.


86. Kanagawa T, Tomimatsu T, Hayashi S, Shioji M, Fukuda H, Shimoya K, et al. The effects of repeated corticosteroid administration on the neurogenesis in the neonatal rat. Am J Obstet Gynecol 2006;194:231–238.


87. Ahlbom E, Gogvadze V, Chen M, Celsi G, Ceccatelli S. Prenatal exposure to high levels of glucocorticoids increases the susceptibility of cerebellar granule cells to oxidative stress-induced cell death. Proc Natl Acad Sci U S A 2000;97:14726–14730.



88. Noguchi KK, Lau K, Smith DJ, Swiney BS, Farber NB. Glucocorticoid receptor stimulation and the regulation of neonatal cerebellar neural progenitor cell apoptosis. Neurobiol Dis 2011;43:356–363.



89. Bhatt AJ, Feng Y, Wang J, Famuyide M, Hersey K. Dexamethasone induces apoptosis of progenitor cells in the subventricular zone and dentate gyrus of developing rat brain. J Neurosci Res 2013;91:1191–1202.


90. Kim JW, Kim YJ, Chang YP. Administration of dexamethasone to neonatal rats induces hypomyelination and changes in the morphology of oligodendrocyte precursors. Comp Med 2013;63:48–54.


91. Gumbinas M, Oda M, Huttenlocher P. The effects of corticosteroids on myelination of the developing rat brain. Biol Neonate 1973;22:355–366.


92. Antonow-Schlorke I, Helgert A, Gey C, Coksaygan T, Schubert H, Nathanielsz PW, et al. Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet Gynecol 2009;113:142–151.


93. Tsuneishi S, Takada S, Motoike T, Ohashi T, Sano K, Nakamura H. Effects of dexamethasone on the expression of myelin basic protein, proteolipid protein, and glial fibrillary acidic protein genes in developing rat brain. Brain Res Dev Brain Res 1991;61:117–123.


Fig. 1
Immunofluorescent staining for myelin basic protein (MBP) in the forebrain of rats administered saline ((A, ×100, 100 µm) and dexamethasone (B, ×100, 100 µm) at P14, and confocal laser microscopic images of the corpus callosum of rats administered dexamethasone (C, ×400, 25 µm), double-stained on P5 using TUNEL and O4 antibody. Repeated administration of dexamethasone (0.5 mg/kg/day) from P1 to P5 reduced the immunofluorescent expression of MBP at P14 in the forebrain (B), compared with administration of saline (A). The expression of MBP decreased in the corpus callosum (CC), and its overlying supracallosal radiation. Many O4-positive cells with pyknotic TUNEL-positive nuclei (►) indicative of apoptosis were observed in the corpus callosum of rats administered dexamethasone.
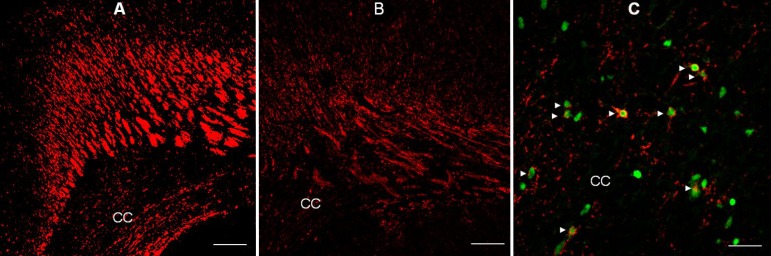
Table 1
Summary for neurodevelopmental follow-up outcomes of randomized controlled trials of postnatal dexamethasone for chronic lung disease in premature infants



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


