Introduction
Partial anomalous pulmonary venous connection (PAPVC) is a rare congenital abnormal cardiac defect in which the pulmonary veins drain into the right atrium (RA) directly, or indirectly by venous connection1). Drainage of the right pulmonary vein into the RA or the superior vena cava (SVC) is the most common type of PAPVC. Since ninety percent of PAPVC are accompanied with atrial septal defect (ASD)2), the echocardiologist should try to find the PAPVC whenever ASD is detected. On the other hand, PAPVC with ventricular septal defect (VSD) is rarely reported3), therefore, when the VSD is initially detected, the echocardiogram may fail to find the PAPVC. We report the first case of a baby who had VSD accompanied by PAPVC, with intact atrial septum in Korea.
Case report
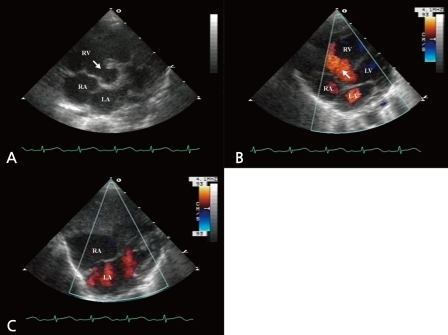
A 2 day-old girl was examined at the Chonnam National University Hospital for the evaluation of a cardiac murmur. She was born by Caesarean section at the local hospital, weighted 3.4 kg at birth, and was suspected to have VSD on antenatal ultrasonography. On physical examination, there were no significant findings apart from a grade 1 systolic murmur on the left lower sternal boarder. Transthoracic echocardiography revealed perimembranous VSD (Fig. 1A-C), and persistent left superior vena cava draining into the RA via the coronary sinus, without bridging the left innominate vein.
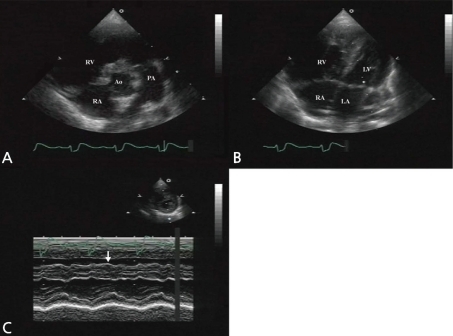
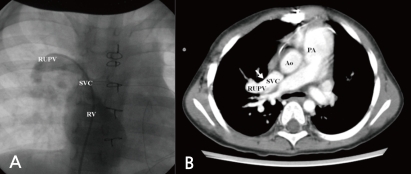
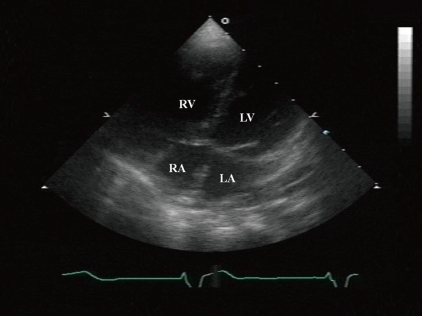
At 5 months old, she was admitted for the symptoms of cough, cold sweating, and tachypnea. On admission day, blood pressure was 110/70 mmHg, body temperature was 36℃, pulse rate was 120 beats per minute and respiration rate was 50 per minute. The chest X-ray showed cardiomegaly with pulmonary congestion. Electrocardiography revealed RA enlargement, and left ventricular hypertrophy. Follow-up echocardiography showed large perimembranous VSD, pulmonary hypertension, and moderate tricuspid regurgitation (TR). However, we could not find either ASD or patent foramen ovale. She had conservative treatment for the symptoms of congestive heart failure, including diuretics and inotropics for 2 weeks, and then she underwent a surgical patch closure of the perimembranous VSD. After the operation, despite of complete closure of the VSD (Fig. 2A), she had suffered from recurrent cough, fever, and pneumonia for 3 years. The chest X-ray showed cardiomegaly and increased pulmonary vascular markings. In addition, the electrocardiogram showed right ventricular hypertrophy, right atrial enlargement, and right axis deviation. The follow-up echocardiography showed both right atrial (RA) and right ventricular (RV) enlargement and right ventricular volume overload (Fig. 2B, C). Then, we performed cardiac catheterization and chest computed tomography at 3 years and 2-months of age. It revealed the right upper pulmonary vein draining into the right SVC, without any evidence of ASD (Fig. 3A, B). At 3 years and 3 months of age, she underwent the modified Warden technique for correction of the PAPVC consisting of the right pulmonary vein connected to the left pulmonary vein. After PAPVC correction, the echocardiography showed disappearance of the right ventricular volume overload (Fig. 4). The follow-up chest X-ray showed improvement of the pulmonary congestion. Since then, she has visited our outpatient department regularly, and her growth and development is according to her age.
Discussion
In the normal heart, there are four pulmonary veins connected to the left atrium which are the upper left, upper right, lower left and lower right pulmonary veins4). Thus, in patients diagnosed with PAPVC, the blood flow from the pulmonary veins returns to the RA instead of the left atrium. The majority of PAPVC has one anomalous pulmonary vein and more than one anomalous vein are also rarely reported. The diagnosis can be missed easily, in up to 25% of patients, the accurate determination of either the number or sites of anomalous connecting pulmonary veins is problematic or impossible5). Therefore, further knowledge of the variation patterns of the pulmonary venous drainage is necessary in order to diagnose PAPVC.
PAPVC is discovered by 0.4 to 0.7% of cases in autopsy series2,6), but this incidence seemed an overestimate because many of these cases were asymptomatic. Thus the true incidence of symptomatic patients is expected to be lower. Moreover, at least one anomalous pulmonary venous connection is present in about three-quarters of the patients diagnosed with asplenia syndrome7). Drainage of the right pulmonary vein into either the RA or the SVC is a frequent type2). Similarly, in the case presented here, the right upper pulmonary vein drained into the right SVC. Up to 90% of the PAPVC cases are accompanied by ASD2). PAPVC from the right pulmonary veins occurs ten times more frequently than the connection initiated from the left pulmonary veins8). Additionally, PAPVC from left pulmonary vein with intact atrial septum is extremely rare8).
Children with PAPVC usually remain asymptomatic and are referred to specialist based on an incidentally noted cardiac murmur5). Symptoms may also occur in older patients and may be secondary to right-sided volume overload or pulmonary vascular obstructive disease. The appearance of the clinical symptoms from PAPVC depends on how many pulmonary veins abnormally return to the RA, and whether the right to left shunt occurs or not9,10). Cardiac arrhythmia detected on a routine clinic visit, an occasionally detected cardiac murmur as well as a widely split second heart sound, can be the first signs of PAPVC5). Some patients complain of tachypnea, subcostal retraction6), repeated pulmonary infections and failure to thrive11,12) or limitation of physical exercise12). On physical examination, marked stridor can be auscultated11). Additionally, in serious cases, fully developed pulmonary edema, hypoxemia, cyanosis and growth failure may appear7). Our case had recurrent pulmonary infection, i.e., pneumonia, due to the right heart volume overload condition.
Imaging examinations to diagnose PAPVC include chest radiography, echocardiography, cardiac magnetic resonance imaging (MRI), cardiac computed tomography, and/or cardiac catheterization. Chest radiographic findings are, as follows: cardiomegaly with prominent RA and right ventricle, dilated pulmonary vessels and pulmonary infiltrated shadow6,12). Echocardiography with color flow mapping reveals pulmonary veins connected either to the RA or the SVC, right heart volume overload, turbulent flow in the SVC, and sometimes TR5,12). Structural MRI is rapidly becoming the procedure of choice for further investigation of the PAPVC5,13).
Even in when echocardiography findings suggest the presence of PAPVC, not all the pulmonary veins may be identified. Therefore cardiac catheterization may be necessary for both the precise anatomic diagnosis and the hemodynamic evaluations. In the clinical case presented here, we confirmed the PAPVC by cardiac catheterization.
The medical treatment of PAPVC is not a routine choice for asymptomatic patients. Patients with heart failure can be managed with diuretics, cardiac glycosides, afterload reduction, and beta blockers. Arrhythmias should be appropriately treated8). Percutaneous transcatheter occlusion of the anomalous pulmonary venous connection using coil was also reported14). The definitive treatment for PAPVC is the surgical repair12, 14). The optimal time for intervention is the preschool age12). The operative technique depends on the number and the site of the anomalous vein or veins. Among several techniques, the Warden method or modified Warden method are usually used15).
Although PAPVC can be isolated, most PAPVC is accompanied with ASD2). Very rarely, PAPVC can be accompanied with VSD, in addition to ASD. Iwasa et al.6) reported a case in which the PAPVC was accompanied by VSD16), ASD, and pulmonary venous stenosis. Kim et al.3) also reported that PAPVC was accompanied by VSD, ASD, and by coarctation of the aorta. Oh et al.17) reported an isolated case of PAPVC, without either ASD or VSD. To the best of our knowledge, the case presented here, i.e., of a PAPVC with VSD but without ASD, is the first case in Korea. When the VSD was initially detected, the PAPVC diagnosis was missed.
In conclusion, we believe that the PAPVC diagnosis should be considered every time a patient presents with right ventricular volume overload without ASD.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


