Timing of parenteral nutrition initiation in critically ill children: a randomized clinical trial
Article information
Abstract
Background
The optimal timing of parenteral nutrition (PN) initiation in critically ill children remains controversial.
Purpose
To identify the optimal timing of PN initiation in critically ill children.
Methods
This randomized clinical trial was conducted in the pediatric intensive care unit (PICU) of Menoufia University Hospital. A total of 140 patients were randomized to receive early or late PN. The early PN group consisted of 71 well-nourished and malnourished patients who received PN on the first day of PICU admission. Malnourished (42%) and well-nourished children randomized to the late PN group (42%) started PN on the fourth versus seventh day after admission, respectively. Mechanical ventilation (MV) was the primary outcome, while PICU length of stay and mortality were secondary outcomes.
Results
Patients who received early PN started enteral feeding significantly earlier (median, 6 days; interquartile range, 2–20 days) than those not provided early PN (median, 12 days; interquartile range, 3–30 days; P<0.001) and had a significantly lower risk of feeding intolerance (5.6% vs.18.8%, P=0.035). The median time required to obtain full calories enterally was shorter in the early versus late PN group (P=0.004). Furthermore, patients in the early versus late PN group had a significantly shorter median PICU stay (P<0.001) and were less likely to require MV (P=0.018).
Conclusion
Patients who received early PN had a lower MV need and duration than those who received later PN and had more favorable clinical outcomes in terms of morbidity.
Key message
Question: What is the ideal initiation timing of parenteral nutrition for critically ill children?
Finding: This randomized clinical trial of 140 children examined the effects of an early or late start of parenteral nutrition on mechanical ventilation need (primary outcome) and length of stay and mortality (secondary outcomes).
Meaning: Children who received early versus late parenteral nutrition had lower mechanical ventilation need and duration.
Graphical abstract. MV, mechanical ventilation; PICU, pediatric intensive care unit.
Introduction
In the pediatric intensive care unit (PICU), nutrition is paramount to patient care. Critically ill infants and children may have a higher metabolic demand, putting them at risk for nutritional deficiency during their illness [1]. Despite recommendations for adequate nutrition, surveys indicate that malnutrition is still an issue in critically ill patients, with rates ranging from 25% to 45% [2].
Nutritional assessment upon PICU admission is recommended in the guidelines of the American Society of Parenteral and Enteral Nutrition to identify at-risk children and guide nutritional support in the PICU [3]. However, controversy persists regarding the optimal means of providing nutritional support. Many standards indicate that early enteral feeding is believed to provide superior clinical outcomes to parenteral nutrition (PN) [4]. Current worldwide feeding guidelines, based on clinical studies with surrogate outcome measures and expert opinion, are still ambiguous regarding when to initiate PN in critically ill children [5]. These children, however, frequently fail to fulfill their energy needs via enteral feeding for reasons such as gastrointestinal intolerance or surgery, and in those children, a PN approach is necessary [6].
The ESPGHAN/ESPEN/ESPR/CSPEN guidelines on PN in critically ill children recommend withholding PN for the first week of admission, and they advise micronutrient supplementation during this time window [7]. Pediatric early versus late parenteral nutrition in critical illness (PEPaNIC) randomized control trials (RCTs) have clarified that delaying PN until after 1st week in intensive care (late PN) was better than early PN [8]. The optimum time of initiating PN remains vague, so the current study aimed to assess the optimum time of PN initiation among critically ill children admitted into the PICU.
Methods
A single-center, open-label randomized controlled trial was conducted at a PICU in a tertiary care hospital from July 2021 to June 2022.
1. Inclusion criteria
The criteria included patients who were critically ill children, admitted to the PICU aged 1 month to 16 years and unable to receive their entire caloric requirements via the enteral route.
2. Exclusion criteria
Children expected to stay in the PICU<3 days (e.g., diabetic ketoacidosis patients), declined to participate in the study and aged less than 1 month or more than 16 years.
3. Data collection
Patients were assessed upon admission via a thorough medical history, clinical examination, and blood and serum analyses. The pediatric sequential organ failure assessment (pSOFA) score was used to measure the severity of the clinical condition at admission and to assess organ dysfunction and depending on the patient's baseline risk level [9]. The pediatric risk of mortality (PRISM) [10] was assessed at the end of the first 24 hours for each patient, using 14 clinical and laboratory variables where data were entered on the website: http://www.sfar.org/scores2/prism2.php that automatically calculates the expected death rate. Early or late PN was given to the patients at random.
4. Randomization, allocation, and concealment
Patients were randomized to early PN and late PN groups using computer-generated random numbers. The patients and the treating physicians were blinded to the type of parenteral fluids administered. The early PN group, which included well-nourished and malnourished children, was given PN on the first day of PICU admissions. Children randomized to the late PN group, children who identified as malnourished, were started on PN on the fourth day and well-nourished were initiated on the seventh day.
5. Methodology
The PN solution contained glucose, protein, electrolytes (sodium, magnesium phosphorus, calcium, and potassium), water-soluble vitamins, trace elements, and amino acids. The PN dose and composition conformed to the local protocol (shown in Supplementary Tables 1–3) [11]. In the early PN group, we included 10%–12.5% glucose (as D-glucose) starting on PN administration day 1. We increased the glucose proportion by 2.5% per day (2.5g/kg/day) until glucose was ~50% to 60% of total kcal consumed at a glucose infusion rate (GIR) of 4–8mg/ kg/min with a maximum GIR of 12mg/kg/min. The glucose level was maintained at 80–150 mg/dL. If hyperglycemia occurred, the GIR was decreased. If hyperglycemia persisted after GIR decrease, insulin was given until the blood glucose level becomes lower than 150 mg/dL was achieved. If hypoglycemia (<80 mg/dL) developed, we increased GIR or the concentration of infused glucose. Protein (Aminoven infant 10%) was administered from day 1 at a dose of 1g/kg and increased daily by 0.5g/kg to reach 3.5g/kg. Electrolytes were also administered from day 1, in the following doses: sodium at 3–5 mEq/kg, potassium at 2–4 mEq/kg, calcium (calcium gluconate) at 0.5–2 mEq/kg, and magnesium (magnesium sulfate) at 2–10 mEq (0.25–1.25 g) daily. Phosphorus (Glycophos) was administered at a dose of 1–1.5 mmol/kg/day. Trace elements were provided via Peditrace administration. The dose of Peditrace in infants and children weighing up to 15 kg was 1mL/kg/day, and in children weighing more than 15 kg, 15mL/day. Water-soluble vitamins were provided by Soluvit N administration at a dose of 1mL/kg in children weighing less than 10 kg and 1 vial/day in children weighing more than 10 kg. Fat-soluble vitamins were provided by administration of Vitalipid Novum Infant at a dose of 1mL/kg/day. Lipids were administered; Smoflipid was started on day 2 at a dose of 0.5g/kg and increased daily by 0.5g/kg to reach 3g/kg/day.
Well-nourished children in the late PN group received a mixture of 5% glucose and saline until the seventh day after admission, and malnourished children received that mixture until the fourth day. To prevent the refeeding syndrome, late PN patients received intravenous trace elements, minerals, and vitamins starting on day 1 and continued until full enteral nutrition [8,12]. PN was discontinued when more than 80% of the nutritional requirement was tolerated enterally. In both groups, enteral nutrition was initiated as soon as possible and gradually advanced up to the target as tolerated.
The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) defined pediatric malnutrition in the clinical setting as “an imbalance between nutrient requirements and intake that results in cumulative deficits of energy, protein, or micronutrients that may negatively affect growth, development, and other relevant outcomes.” [13] For each patient admitted, the admission weight and length/height were measured. If the weight-for-length z score was ≤-2 standard deviation, the patient was considered malnourished using World Health Organization scale [14].
6. Criteria for the introduction of enteral feeding
Enteral nutrition was initiated and progressed gradually in parallel with a gradual reduction of PN. The decision to start enteral feeding was initiated when the patient showed (1) hemodynamic stability, (2) lack of significant respiratory distress, and (3) audible intestinal sounds, no abdominal distention, and normal gastric residual.
The enteral feeding route used was in the form of nutrition administered through the mouth (oral feeding) or through a tube directly connected to the stomach, called nasogastric tube feeding. We typically use a standard polymeric formula feed, advancing to full calories at 10–20 mL/hr [15] and administering continuously or intermittently.
Patient weight was also monitored twice weekly throughout their PICU stay. Blood glucose was measured 4 hours after starting or changing the glucose concentration of infusate, then daily for 2 days or until the patient was stable. Serum electrolytes, bicarbonate, and blood urea nitrogen (BUN) were measured twice daily after starting or changing the infusion rate or composition, then twice weekly. Liver function tests, albumin, phosphate, magnesium were measured initially, then weekly unless the patient was unstable or warranted closer monitoring (e.g., refeeding syndrome). Finally, serum triglycerides (if fat emulsion in use) were measured daily after starting or when changing the quantity of fat administration, then weekly. Patients were followed up until discharge from PICU or death.
7. Outcome endpoints
The primary outcome was the need for mechanical ventilation (MV). The secondary outcomes included (1) PICU stay (among survivors); (2) vasoactive-infusion days; (3) incidence of new infections acquired throughout the course of receiving health care that was not present at the time of admission are known as healthcare-associated infections; 2 examples of which include ventilator-associated pneumonia, occurring 48 hours after endotracheal intubation [16], and central line-associated bloodstream infection (CLABSI) of a central venous catheter (CVC) [17]; and (4) PICU mortality.
8. Sample size estimation
Based on the primary outcome of the study to detect need for MV and based on review of past literature [18], who found that, there was significant relation between need for MV and nutrition feeding; need for MV was lower in children receive nutrition feeding than those do not receive 31.7% versus 68.3 respectively. Sample was calculated by the following equation {n=P1 (1-P1)+P2 (1-P2) / (P1–P2)2* C} where n=desired sample size, P1, 2=the proportion in each group and C=standard values of α (with β equal to 7.85 at 80% power) at a confidence level of 95%. The minimum calculated total sample size required after adding a dropout rate of 10% was 82 participants. Due to availability of cases during our practical part of the study sample was increased to 140 participants which were allocated by randomization into 2 group early PN (n=71) and late PN (n=69).
9. Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the Menoufia University and Faculty of Medicine (approval number: 3/2021 PEDI 3-2). Informed consent was obtained from the parents (or legal representative).
10. Statistical methods
The IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA) was used to perform all statistical calculations. Numbers and percentages were used to represent categorical variables. Quantitative variables were presented as the median (range) with a skewed distribution and compared using the MannWhitney U test (for 2 groups) or the Kruskal-Wallis test (for 3 or more groups). The association between categorical variables was assessed using chi-square, yet Fisher exact test was employed when more than 25% of the cells had anticipated counts of less than 5. Multivariate regression analysis was used to detect independent predictors for MV need. The threshold for statistical significance was defined as P<0.05.
Results
1. Characteristics of the study population
Two hundred critically ill children were assessed for eligibility, of which 60 children were excluded. The remaining 140 children were randomized to receive either early or late PN. There were 71 patients in the early PN group and 69 in the late PN group; the CONSORT (consolidated standards for reporting of trials) 2010 flow diagram of the current trial is shown in Fig. 1.
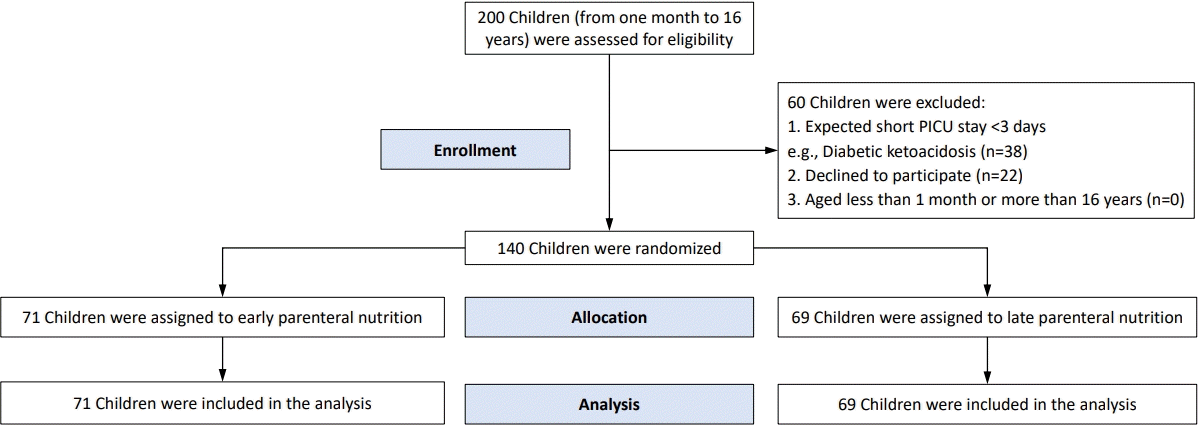
CONSORT (consolidated standards for reporting of trials) 2010 flow diagram of the current trial. PICU, pediatric intensive care unit.
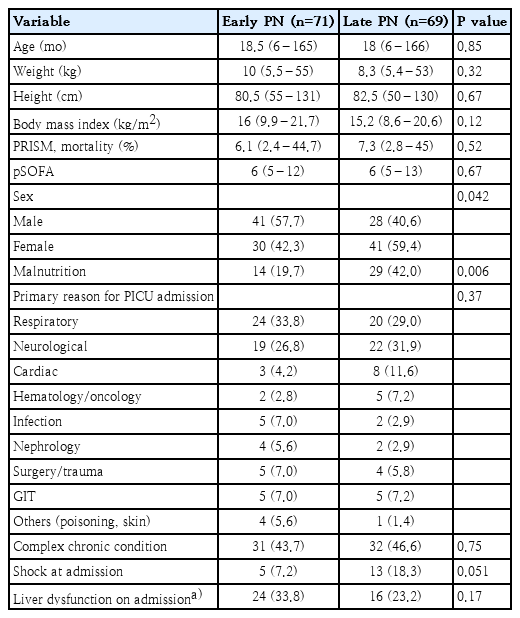
The baseline characteristics of both groups are shown in Table 1. No significant difference was found in illness severity between the 2 groups on admission as assessed by pSOFA and PRISM scores.
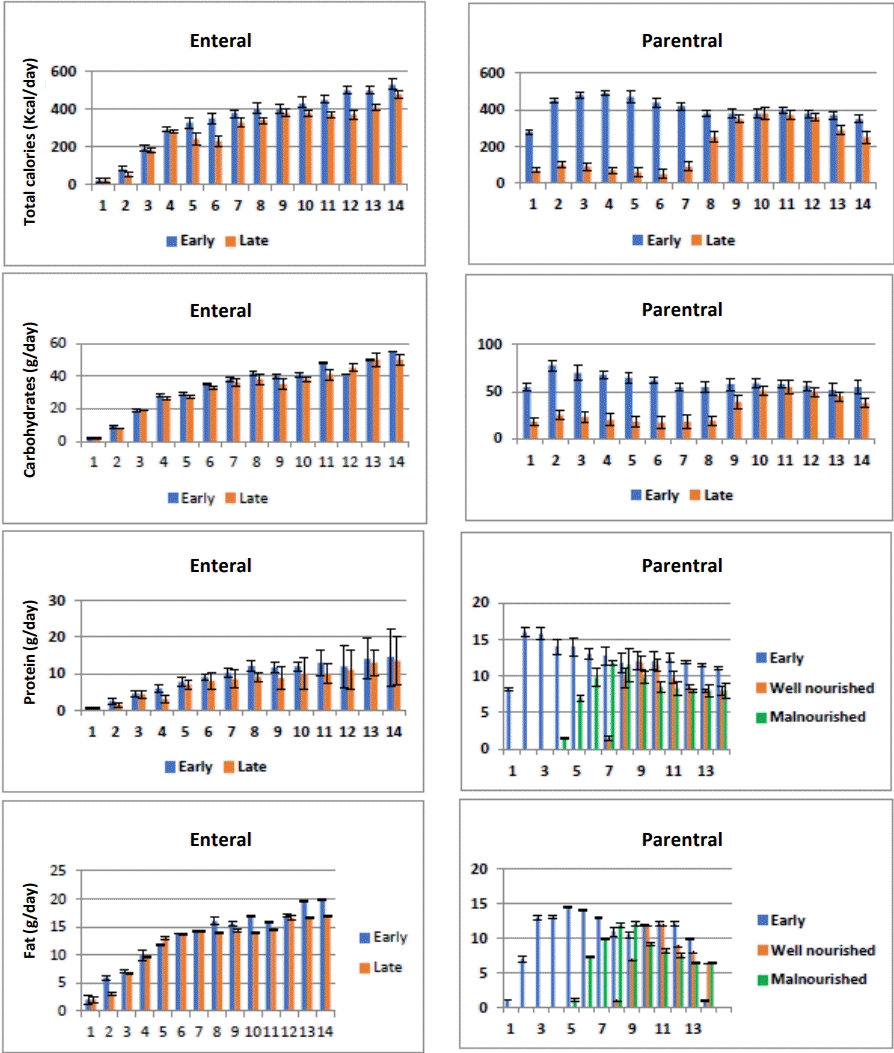
Daily caloric and macronutrients intake from PICU admission until day 14 (total caloric, carbohydrate, protein, and lipid intake) for enteral and parenteral routes in both groups are shown in Fig. 2.
2. Nutritional data of patients
Table 2 shows the nutritional data of patients. Patients given early PN were able to start enteral feeding earlier than those provided late PN (median, 6 days; interquartile range [IQR], 2–20 days vs. median, 12days; IQR, 3–30 days; P<0.001), had a lower risk of feeding intolerance (5.6% vs.18.8%, P=0.035) and required less time to reach full calories enterally (median, 8 days; IQR, 7–10 days vs. median, 12.5 days; IQR, 11–20 days; P=0.004). There was no significant difference between the 2 groups in the total duration of PN (P=0.78).
3. Outcome of the study population
Patients with early PN had lower need and shorter duration of MV with more MV-free days (P=0.018, P<0.001, P=0.001, respectively). There was no significant difference in mortality between the early and late PN groups (P=0.21). Patients with early PN were more likely to have shorter PICU stays (median, 8 days; IQR, 6–55 days vs. median, 16.5 days; IQR, 7–75 days; P<0.001) and has shorter free PICU stays (median, 9 days; IQR, 8–29 days vs. median, 15 days; IQR, 10–37 days; P=0.033). They were also more likely to experience fewer vasoactive-infusion days (P=0.013). Patients receiving early PN had fewer CVC days and lower nosocomial infection rates than late PN patients (P=0.001) (Table 3).
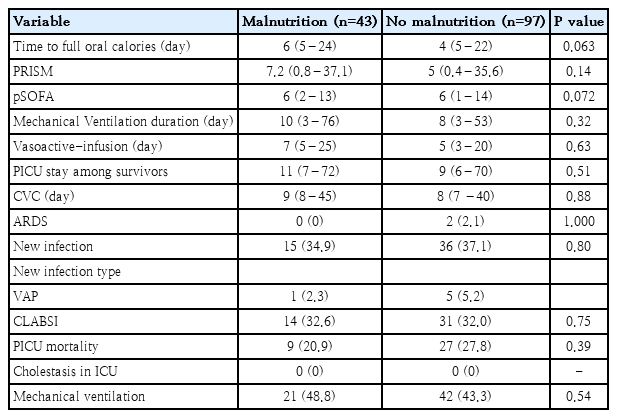
Accordingly, the clinical outcome of patients with and without malnutrition was not significantly different regarding illness severity, as assessed by PRISM and pSOFA. No significant difference was found between the 2 groups regarding disease outcome, including mortality, PICU stay, and the incidence of acute respiratory distress syndrome, MV duration, vasoactive-infusion days or incidence of new infection (Table 4).
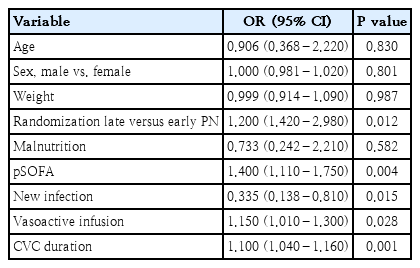
Multivariate regression analysis revealed that randomization to late PN compared with early PN was an independent predictor for MV need. Also, pSOFA score, presence of new infection, increased duration of vasoactive drugs, and CVC are independent predictors for MV need (Table 5).
Discussion
Over time, children admitted to the PICU who are critically ill are at risk of changing their nutritional status and anthropometric measures [19,20]. Inadequate nutrition therapy for critically ill patients has a higher risk of morbidity and mortality. So, nutritional therapy for these patients is essential, especially for those with prolonged PICU stays [21].
We assessed illness severity by PRISM and pSOFA was not significantly different between the 2 groups, indicating that this was not a confounding factor.
Data analysis revealed that patients who received early PN had improved outcomes compared to those with late PN. This was demonstrated by a decrease in length of stay, MV need and duration, and infection rates.
We chose not to exclude the malnourished group because it represents a high percentage of late PN population (42%). This disparity in feeding timing in the late PN group is because malnourished children have low energy stores, and 6 days of inadequate nutrition may be too long for these children even though it may not be too long for well-nourished children. Our results indicate no significant difference between patients who had malnutrition at admission and those who were not malnourished, suggesting that adequate nutritional management at the PICU can overcome the effect of chronic malnutrition and indicate that malnutrition was not a risk factor for mortality or morbidity. This surprising result can be explained based on the greater care provided by doctors to such children, suggesting that malnutrition is a modifiable factor that can be substantially overcome by greater alertness on the part of physicians. In addition, our sample size might not have been large enough to detect a significant difference in mortality outcomes.
This result came in line with Nangalu et al. [22], who stated that the mortality in children with borderline malnutrition was slightly less than the children with normal nutrition (7% vs. 11 %) but this difference was not statistically significant. There was no statistically significant difference in PRISM score among cases and controls and also in mild to moderately nourished to severely malnourished children. Another study showed that malnutrition is common among children admitted to an ICU. This factor was not a predictor of mortality but showed an independent association with the length of MV [23].
Patients given early PN were able to start enteral feeding earlier, had a lower risk of feeding intolerance and required less time to reach full calories enterally. There was no significant difference in the total duration of PN between the 2 groups. There was also no significant difference in mortality between the 2 groups in the current study, and the frequency of early PN was reduced in nonsurvivors. Patients with early PN had a shorter PICU stay, lower vasoactive-infusion days and lower need and shorter duration of MV.
In our result, early PN was associated with shorter duration of MV. It has been previously found that undernutrition plays a vital role in ventilator-induced diaphragmatic weakness and respiratory muscle dysfunction which may cause delaying in MV weaning [24,25]. However, we found no significant difference between children with malnutrition and those without malnutrition, so we can be explained our results by giving good nutritional support at PICU can get over chronic malnutrition effect; particularly, supplementation of phosphorous and potassium at the PICU can decrease hypokalemia and hypophosphatemia incidence which are risk factors for extubation failure. Fivez et al. [8] reported that MV support duration was shorter among patients receiving late PN than among those receiving early PN.
Patients with early PN had lower CVC days and fewer nosocomial infections than those given late PN. In critically ill children, a late-initiation supplementary PN method increases the risk of nosocomial infection compared to an early-initiation approach [5]. In children, CLABSI is a common PN complication. The PN administration and the CVC presence are independent risk factors for developing sepsis [26]. Periods of prolonged fasting can result in intestinal mucosa atrophy, leading to gut-related sepsis [27].
The use of more than one CVC simultaneously and a longer period of CVC use have been observed as key risk factors for CLABSI in the PICU [28], and CLABSIs are associated with increased morbidity, length of stay, and hospital costs [29].
In a previous study, patients in the PICU who needed inotropes were at 10 times increased risk of mortality than those who did not need inotropes [30]. In addition, administration of more than one type of inotrope was significantly associated with death [31].
A previous study [8] showed that patients with late PN had a 10.7% new infection rate, compared to 18.5 percent for children who received early PN. In children who received late PN, the duration of PICU stay was 6.5±0.4 days compared to 9.2±0.8 days for those receiving early PN. In children receiving late PN, there was also a higher rate of earlier discharge from the PICU at any time. Children receiving late PN required a shorter MV support period than early PN children (P=0.001) and a shorter hospital stay (P=0.001). The late PN group had lower plasma levels of gamma-glutamyl transferase and alkaline phosphatase (P=0.001 and P=0.04, respectively), as well as higher levels of bilirubin (P=0.004) and C-reactive protein (P= 0.004) than the early PN group (P=0.006).
Previous studies [32,33] reported that regardless of age, the severity of illness, diagnosis, or risk of malnutrition, withholding PN in the first week of the PICU stay reduced the incidence of new infections and permitted faster recovery than early PN supplement within 24 hours after PICU admission. Late PN lowered the absolute risk of new infections and reduced the period of stay in the PICU among undernourished patients. Weight deterioration during PICU stay was associated with worse clinical outcomes, according to a larger longitudinal study of all PEPaNIC patients with weight z scores available upon admission and on the last day in the PICU. However, withholding supplemental PN during the first week did not affect weight z score deterioration during the PICU stay [34].
In a retrospective single-center investigation, late PN initiation was related to a higher nosocomial infection rate than early PN initiation [5]. A large multicenter randomized controlled trial demonstrated that independent of age or nutritional status, withholding PN supplements for the first week in the PICU was clinically superior for the short-term outcome than initiating PN supplements within 24 hours of admission [35]. Administration of amino acids may explain how early PN initiation negatively influences the liver and kidneys by suppressing necessary autophagy activation and shuttling amino acids to urea synthesis [36].
Our results showed that patients with early PN had a more favorable clinical outcome in terms of morbidity; this result is supported by a faster initiation of EN in early PN patients driven by the lower illness severity. Enteral feeding may induce a minimal process known as trophic feeding, can increase gut barrier function, begin the release of enteral hormones, and promote intestinal perfusion [37].
The current RCT in critically ill children showed that the early PN improved outcomes more than late PN administration. The European and American nutrition guidelines recommend early PN in hospitalized children, especially those that are very young, to prevent/correct malnutrition and sustain appropriate growth when enteral nutrient supply is insufficient [3,38]. General pediatric guidelines advise PN supplement initiation when children with malnutrition or low birth weight are expected to be on limited enteral nutrition for more than 3–5 days, and well-nourished children, more than 5–7 days [39,40].
In conclusion, in critically ill children, early PN correlated to lower MV need and duration than those who received later PN. There was no change observed in overall mortality. We believe this is related to earlier initiation of enteral nutrition, which leads to lower illness severity overall.
A high malnutrition rate would likely not permit our results to be generalizable to other centers. Further research, in the form of multicenter RCTs, is warranted to determine the optimal composition of supplemental PN in critically ill children. A nutritional plan must be individualized for each patient according to nutritional status, disease severity, and patient tolerance.
Supplementary materials
Supplementary Tables 1-3 can be found via https://doi.org/10.3345/cep.2023.00178.
Daily parenteral nutrition of energy, fluid, macronutrients, and electrolyte requirements
Daily parenteral nutrition of vitamins requirements
Daily parenteral nutrition of trace element requirements
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Trial registration
Registered on January 5, 2021, at https://clinicaltrials.gov/ct2/show/NCT04693143. ClinicalTrials.gov Identifier: NCT04693143.
Author Contribution
Conceptualization: NYS, AAM; Data curation: NYS, AAM; Formal analysis: NYS, AAM; Funding acquisition; NYS, HMA, AAM, NBA; Methodology: NYS, AAM, NBA; Project administration: NYS, AAM, NBA, HMA, MIG; Visualization: NYS, NBA, AAM; Writing-original draft: NYS, AAM; Writing – review & editing: NYS, AAM
Acknowledgements
The authors express their gratitude to the participants who participated in the study.