Effects of probiotics combined with dietary and lifestyle modification on clinical, biochemical, and radiological parameters in obese children with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: a randomized clinical trial
Article information
Abstract
Background
Childhood obesity is a global problem associated with metabolic abnormalities. The gut-liver axis is thought to play a major role in its pathogenesis. Probiotics are known to alter the gut microbiota and, therefore, could be a therapeutic option in the management of childhood obesity-related complications.
Purpose
This double-blind randomized placebo-controlled trial evaluated the effects of probiotics on metabolic derangement in obese children with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH).
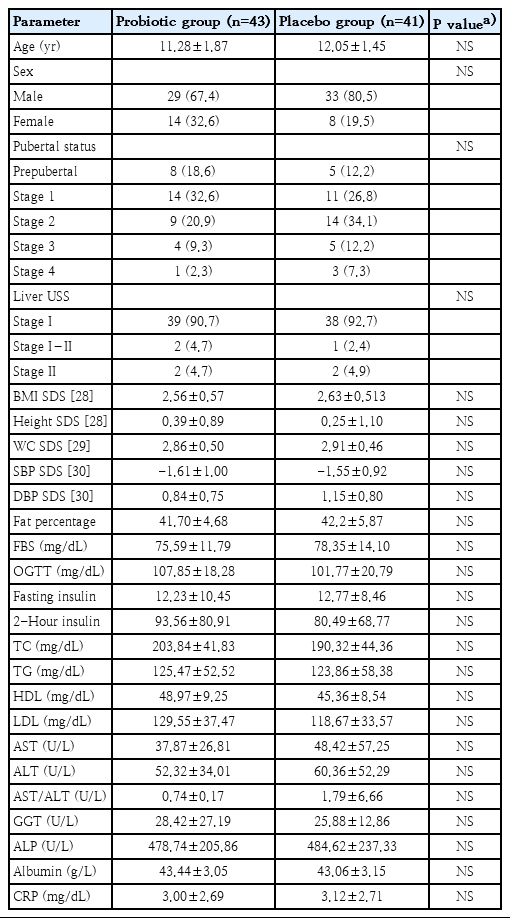
Methods
Obese children with NAFLD/NASH treated at the nutrition clinic of the University Paediatric Unit at Lady Ridgeway Hospital, Colombo, were recruited. Anthropometry, body fat, metabolic derangement, and liver ultrasound scan (USS) results were evaluated at baseline and after 6 months. Transient elastography (FibroScan) was performed on a subsample of these patients. Eighty-four patients were recruited and randomized into the probiotics (n=43) and placebo (n= 41) groups. The mean age was 11.3±1.9 versus 12.1±1.5 years in the probiotic and placebo groups, respectively. Baseline parameters including liver disease stage on USS, body fat percentage, fasting blood sugar, lipid profile, liver function, and C-reactive protein showed no significant intergroup differences.
Results
In the probiotic group, a statistically significant reduction in body mass index was noted from the baseline value. However, the reduction was not significant compared with the placebo group. There was a significant reduction in triglycerides, aspartate transaminase (AST), alanine aminotransferase (ALT), AST/ALT ratio, and alkaline phosphatase in the placebo group over the treatment period. Although the liver disease stage on USS improved from stage II–III to stage I in a small number of patients in the probiotic-treated group, transient elastography performed in a subsample did not demonstrate significant improvement in either group.
Conclusion
Our results indicate that probiotics have no advantage over lifestyle modification for improving obesityassociated metabolic derangement in children.
Key message
Question: Could probiotics be used as a therapeutic modality in nonalcoholic fatty liver disease/nonalcoholic steatohepatitis?
Finding: There seem no added advantages over lifestyle modifications compared to Probiotics.
Meaning: There does not seem to be an advantage of probiotics over lifestyle modifications in improving obesity-associated metabolic derangement in children.
Introduction
Childhood obesity is a global health problem, which leads to metabolic derangements including insulin resistance, metabolic syndrome, impaired lipid metabolism, and nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). Hence, prevention of obesity in the younger generation is of paramount importance. However, over the years most preventive methods have failed to decelerate the rapid growth of this health burden. Therefore, it is important to find new therapeutic options which could be used in addition to lifestyle modifications.
It is documented that NAFLD prevalence in children varies from 3% in the general population to 80% in obese children [1]. In children, NAFLD is commoner among males, during puberty and is associated with insulin resistance [2]. Several studies have estimated that NAFLD/NASH would increase the 5-year medical costs by 26% [3]. In Sri Lankan children, the prevalence of NAFLD in a suburban community was 8.4% [4], and the prevalence of presumed NASH was estimated to be about 18% among obese children [5].
The pathogenesis of NAFLD is unclear. Theories regarding its development are based on the ‘2-hit hypothesis,’ where the ‘first hit’ involves hepatic lipid accumulation, and insulin resistance is proposed to be the main contributing factor for the development of steatosis [6]. Then, oxidative stress followed by lipid peroxidation as well as the action of proinflammatory cytokines (e.g., tumor necrosis factor-alpha [TNF-α]), adipokines, and mitochondrial dysfunction initiate the ‘second hit,’ which leads to the progression of simple steatosis to NASH [7]. In addition, Dowman et al. [8] recently described a ‘third hit,’ which is also caused by oxidative stress that inhibits the replication of mature hepatocytes resulting in an increased number of hepatic oval cells.
It has been reported that NAFLD might be linked to small intestinal bacterial overgrowth (SIBO), which is defined as an increase in the number and/ or alteration in the type of bacteria in the upper gastrointestinal tract owing to the loss of more than one of the several endogenous mechanisms. SIBO induces liver injury by gut-derived lipopolysaccharides (LPS) and TNF-α production leading to steatohepatitis [9,10]. Solga and Diehl concluded that bacterial overgrowth, release of the LPS constituent of the gramnegative bacteria, and impaired intestinal barrier integrity results in increased endotoxin absorption subsequently leading to liver toxicity [11]. This theory is supported by several studies. One of the studies by Madsen et al. has shown that SIBO is present in 50% of patients with nonalcoholic steatosis [12].
Probiotics are live organism that when consumed in adequate quantities, confer a health benefit to the host (World Health Organization, WHO). They exert their anti-inflammatory effects through several mechanisms including intestinal barrier stabilization, immunomodulation, and SIBO alteration, that can contribute to the clinical benefits in obesity-related metabolic complications [12-17].
Children with NAFLD are often asymptomatic or have nonspecific symptoms. Although there are no specific biochemical tests describing hepatic steatosis on imaging, an AST/ALT ratio of less than 1 suggests the diagnosis of NAFLD, with or without the development of hepatic fibrosis [18]. Abdominal ultrasound scan (USS) is often used in screening for NAFLD as it has a predictive value of 84%–94%, but USS cannot detect a fat load less than 30% in the liver, compared to histological examination [19]. However, recent large prospective pediatric cohort showed a good correlation between steatosis score assessed by USS and the severity of steatosis on liver biopsy, which is the gold standard study to diagnose NAFLD/NASH [20]. However, liver biopsy is invasive, has a potential for sampling errors and inconsistent interpretation of the histopathology. Furthermore, recent studies demonstrate the benefits of the transient elastography to detect liver fibrosis in the pediatric age as a noninvasive method [21]. However, elastography is prone to fail in obesity, presence of ascites, liver congestion, and with narrow intercostal spaces [21]. But usage of algorithms such as the controlled attenuation parameter (CAP) helps to minimize these errors [22].
At present, there are limited management options to tackle obesity-related metabolic derangements apart from losing weight through dietary modification and physical activity [23]. Unfortunately, the target of gradual and controlled weight loss is difficult to achieve by diet and physical exercise. An extremely low percentage of individuals are able to steadily lose weight through regular exercise and dietary modifications [24], warranting new therapeutic approaches. Considering the evidence of the possible role of gut microbiota in the development of obesity-related metabolic derangements, probiotics may be utilized to modify gut microbiota as a preventive or therapeutic strategy.
Malasanos and Stacpoole [25] show that probiotics enhance the barrier function of epithelial cells and decrease intestinal permeability and endotoxemia in patients with liver disease. Ma et al. [26] showed that probiotic therapy significantly decreased alanine aminotransferase (ALT), aspartate transaminase (AST), total cholesterol, high-density lipoprotein, TNF-α, and improve insulin resistance in NASH patients. Also, a placebo-controlled randomized study in histologically confirmed cases of NAFLD treated with daily Lactobacillus bulgaricus and Streptococcus thermophiles showed a decrease in ALT and γGT. Another study showed that serum AST, ALT, and ultrasound grading of NASH improved in the group treated with metformin and probiotic compared to the group treated with metformin alone [27].
The increase in the incidence of childhood obesity, and its related metabolic problems, has reached epidemic proportions in developing countries. However, existing medical and nonmedical efforts to tackle this problem are currently inadequate prompting the investigation of safe and inexpensive novel strategies. Despite the limited number of randomized controlled trials, probiotics have shown promising results in treating the metabolic consequences of obesity. Hence, this study attempted to evaluate the effectiveness of probiotics in the treatment of obesity-related metabolic derangement in a group of obese Sri Lankan children with NAFLD/NASH.
Methods
1. Trial design
A double-blind, randomized, placebo control trial.
2. Study population/participants
Children between age 5–15 years of age, with a body mass index (BMI) more than +2 standard deviation for age of WHO standards (2007) together with AST/ALT ratio less than 1 and ultrasound evidence of hepatic steatosis, including grade I to III, were recruited from nutrition clinic conducted by Professorial Paediatric Unit of University of Colombo at the Lady Ridgeway Hospital for Children, Sri Lanka. Children with an acute infection, on long-term medication, chronic illness and on antibiotics within 2 months period of recruitment were excluded after studying past records and clinical evaluation.
3. Intervention
The 2 randomized groups were as follows:
Group 1: structured diet (Supplementary material 1) + physical activity (Supplementary material 2) + probiotics (BioKult 14 strain probiotic capsule – Supplementary material 3)
Group 2: structured diet (Supplementary material 1) + physical activity (Supplementary material 2) + placebo (a capsule without probiotic strains – Supplementary material 3)
The dose was one capsule for children under 12 years and 2 capsules for children above 12 years of age on each day as per manufacturer’s guidance.
Both groups were followed up for 6 months ensuring they adhered to the prescribed diet, physical activity and treatment with compliance chart and direct questioning during the monthly interval follow-ups.
The diet and the exercise schedule were structured. We have regularly checked the compliance along with the medication. However, exact calory count was not carried out due to practical limitations. Also, we checked the compliance with direct questioning.
Both groups were observed for possible side effects. However, none were reported.
4. Outcome assessment
Outcome assessment was done after 6 months by acquiring anthropometric, clinical, biochemical, and radiological parameters similar to baseline assessment.
The primary outcome measures were liver transaminases (AST, ALT), USS assessment of hepatic steatosis, and transient elastographic assessment of liver stiffness and steatosis quantification.
The secondary outcome measures were gammaglutamyltransferase, lipid profile, glucose homeostasis, metabolic syndrome, body fat mass, and anthropometric parameters. A research assistant, a physician, and a radiologist evaluated the patients.
No changes to trial outcomes were done after the trial commenced.
5. Sample size
Sample size was calculated to determine a statistically significant difference in the mean liver function test levels at baseline and after 6 months. Guided by the findings of Aller et al. [27], a standardized effect size of 0.55 was estimated to be seen after 6 months of treatment. Using an α error of 5%, a β error of 20% (power of 80%), and a nonresponse rate of 10%, calculated sample size was 43 subjects per treatment arm.
6. Recruitment and randomization
Informed, written consent was obtained from the guardian and assent from the patient when they were above 12 years of age.
Participants, once registered for the trial were randomly allocated to 2 groups (receiving either probiotics or placebo) using a computer-generated, concealed allocation sequence. Both the subjects and the investigators implementing the protocol were blinded to the treatment.
7. Baseline assessment
Baseline evaluation, comprising anthropometric parameters, body composition measurement using Bio Electrical Impedance (BIA - InBody, Seoul, Korea), blood pressure measurement with sphygmomanometer (with age-appropriate cutoff using standard mercury spigmomanometer) and pubertal staging was conducted by trained research assistants. Blood was collected for glucose, lipid profile, insulin, liver aminotransferase (AST/ALT), gamma glutamyltransferase, alkaline phosphatase, high-sensitive C-reactive protein, and albumin, after 12 hours of overnight fast. Also, random blood sugar and insulin levels were measured 2 hours after 1.75 g/kg (maximum, 75 g) anhydrous glucose challenge. In addition, detailed USS liver was performed on each subject by a consultant radiologist categorizing hepatic steatosis according to National Health and Nutrition Examination Survey III criteria, and in a sub sample (n=27), elastography (Fibroscan, Echosens, Paris, France) was performed.
Additionally, chronic liver diseases in subjects were ruled out by performing hepatitis B surface antigen, hepatitis C antibody, hepatitis A antibody, serum ceruloplasmin, and full blood count (total volume of 10–15 mL of blood was processed). A positive test would have been a criterion to exclude from the study. None were positive in these screening tests.
8. Outcome assessment
Outcome assessment was done after 6 months by acquiring anthropometric, clinical, biochemical, and radiological parameters similar to baseline assessment. The primary outcome measures were liver transaminases (AST, ALT), USS assessment of hepatic steatosis, and transient elastographic assessment of liver stiffness and steatosis quantification. The secondary outcome measures were gammaglutamyltransferase, lipid profile, glucose homeostasis, metabolic syndrome, body fat mass, and anthropometric parameters.
A research assistant, a physician, and a radiologist evaluated the patients.
9. Data analysis
Data analysis was performed by IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) for windows. P value less than 0.05 was considered as significant. Baseline characteristics of the treatment and control groups were compared using chi-square test and independent samples t test or relevant nonparametric tests. Between the 2 groups, the anthropometric, metabolic, and radiological parameters at 6 months as well as the pre-post difference in the parameters were compared using independent samples t test or relevant nonparametric tests. Within the treatment and control groups, the pre-post difference in the parameters were assessed using paired t test or equivalent nonparametric tests. Intention to treat analysis was performed, substituting any missing values with the latest available measurement.
10. Ethical considerations
The study was designed appropriately to ensure scientific validity. Ethical clearance was obtained from Ethics Review Committee of Faculty of Medicine, University of Colombo (EC-16-030) and Lady Ridgeway Hospital for Children.
Permission to conduct the study was obtained from the relevant authorities including Sub Committee on Clinical Trials (SCOCT) of Ministry of Health. The study was registered in the Sri Lankan Clinical Trials Registry (SLCTR/2016/021).
Participation in the study was voluntary. Informed written consent was obtained after providing the necessary information and giving the patients/their guardians’ adequate time and information to make a decision on their own.
Personal details were collected in a separate data sheet that was detachable from the main questionnaire. All hard copies of data were kept under lock and key. The electronic database was password protected. Adequate privacy was maintained during history taking and all physical examination procedures.
Results
Eighty-four obese children with NAFLD/NASH were randomized into probiotics group (n=43), who received structured diet plan and physical activity plan together with probiotic treatment according to the age or the control group (n=41) who received the placebo treatment in addition to similar diet and physical activity plan (Fig. 1, Table 1).
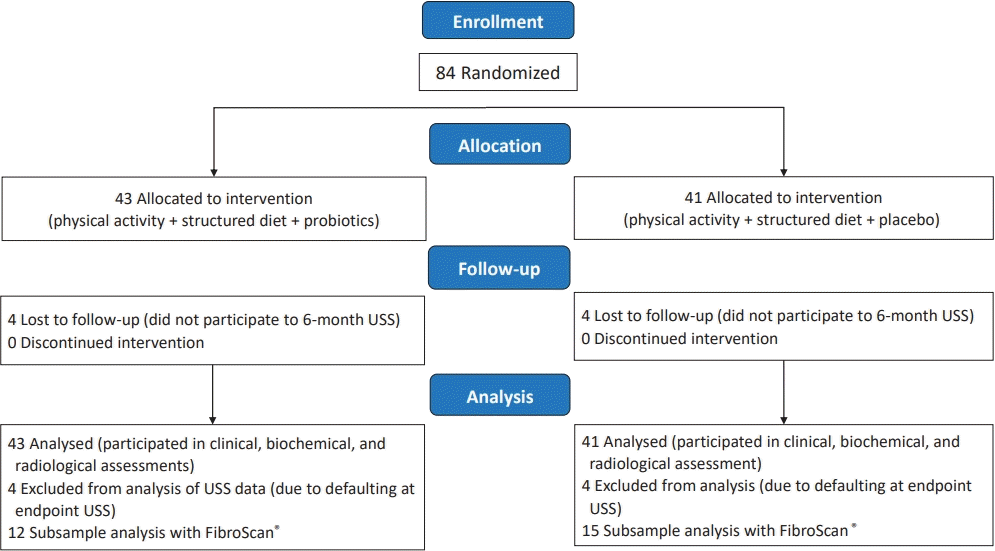
Participant flow diagram. The 2 groups were similar in age, sex, and pubertal stage distribution. Table 1 summarizes the baseline anthropometric characteristics, body composition, metabolic, and ultrasound-related characteristics of the 2 study groups, and there were no statistically significant difference in their baseline values.
After the study period of 6 months probiotic and placebo treated groups showed significant reduction of BMI compared to baseline values (P=0.023 and P=0.001 respectively). However, there was no significant difference in BMI between the probiotic and placebo groups. The placebo group showed significant improvements in serum triglycerides, AST, ALT, AST/ALT ratio, and alkaline phosphatase from baseline values. The probiotic group did not show such changes in biochemical parameters. However, the placebo did not demonstrate a significant advantage over probiotic-treated group (Table 2).
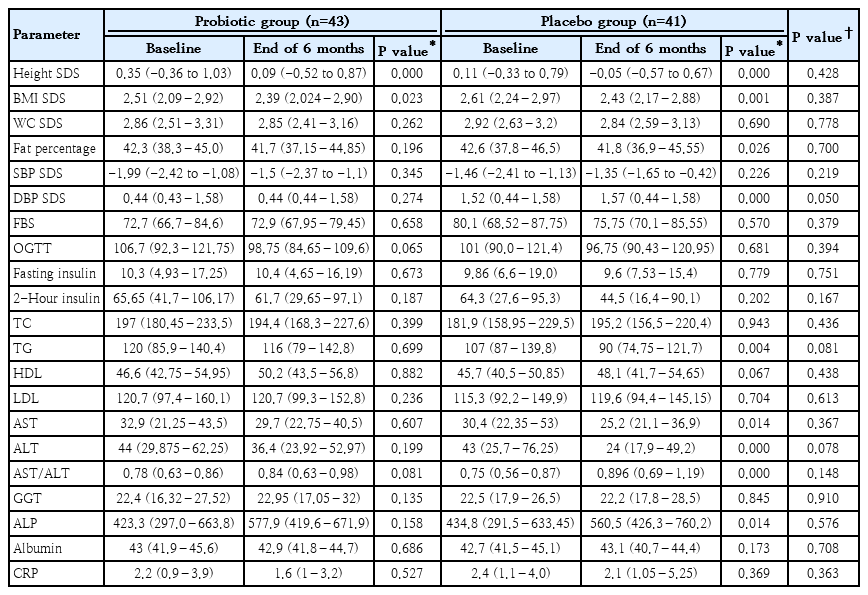
Median anthropometric and metabolic parameters at baseline and 6 months later by treatment arm (N=84)
However, other metabolic parameters including, fasting blood sugar, oral glucose tolerance test, fasting insulin together with post prandial insulin, total cholesterol, high-density cholesterol, and low-density cholesterol did not demonstrate statistically significant improvement from baseline in either group. Furthermore, clinical parameters including waist circumference and body fat percentage, did not show significant improvement over 6 months.
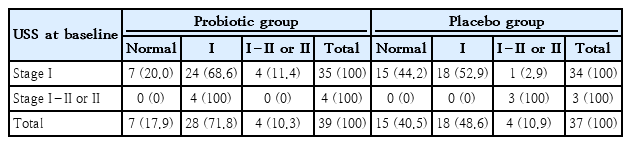
The USS imaging of subjects showed that in the stage I1 fatty liver category, 20% in probiotic arm, and 44.2% in placebo arm, down staging to a normal USS. The probiotic-treated group showed 100% (n=4) conversion of USS stage I–II or II fatty liver to stage I fatty liver by 6 months (Table 3). In the placebo group all (n=3, 100%) who had fatty liver of stage I–II or II at baseline remained at the same stage at the end of 6 months. However, the numbers were too small for statistical significance.
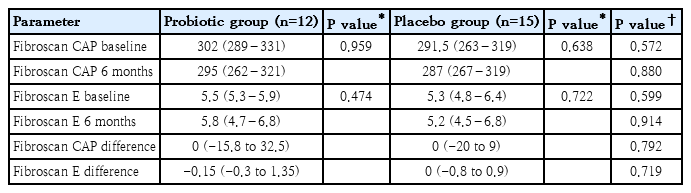
Although USS studies showed some improvement of fatty liver in stage I–II or II with probiotic treatment, the limited subjects (n=27) in both groups who underwent transient elastography did not show statistically significant improvement in fatty liver parameters during the 6 months study period (Table 4).
Discussion
Obesity-related metabolic derangements are reaching epidemic proportion in children worldwide leading to increased morbidity and health cost. The clustering of various cardio metabolic risk factors associated with insulin resistance underlies the concept of metabolic syndrome and is closely related to the increasing levels of adiposity. Also, elevated rates of lipolysis inherent in adipose tissue, alterations in fatty acid fluxes and consequent ectopic fat deposition in skeletal muscle and liver are thought to be the mechanisms linking altered fat distribution and insulin resistance in metabolic syndrome [31].
Out of the metabolic derangements in obesity this research aimed at studying NAFLD/ NASH in view of a novel therapeutic option.
NASH was first described in obese children by Moran et al. and Colab [32,33], after 3 years of describing the condition in adults in 1980. Nonalcoholic fatty liver disease refers to a spectrum of conditions ranging from simple hepatic steatosis to steatohepatitis, which is characterized by hepatic inflammation, liver cell injury, and fibrosis and cirrhosis. From a pathological point of view, NAFLD is where there is excess accumulation of fat in the liver, in the form of triglycerides. Whereas NASH has liver cell injury and inflammation in addition to fat deposition, which can progress to cirrhosis, and sometimes even result in hepatocellular carcinoma. Recent studies describe NAFLD as a hepatic manifestation of the metabolic syndrome [34], highlighting the importance of metabolic complications in obese children.
The concept of using probiotics as a novel therapeutic option in children with NAFLD/NASH is based on theory that SIBO9) leads to the development of liver cell injury based on the 3-hit hypothesis [8].
When the intestinal barrier fails, bacterial translocation will occur, causing endotoxemia especially in the portal circulation initiating a proinflammatory cascade. However, under normal circumstances this endotoxemia is rapidly cleared by the liver’s reticuloendothelial system. But, at times of liver disease or longterm exposure to hepatotoxins, a cascade of morphological and functional changes begins in the liver inducing an acute inflammatory response, which releases reactive oxygen metabolites, proteases, and other enzymes from polymorphonuclear cells resulting in further damage to the liver [35]. Furthermore, it is known that obesity, diabetes, and insulin resistance are associated with low-grade systemic inflammation [36] and metabolic endotoxemia [37].
On the other hand, evidence suggests that children generally show poor compliance with lifestyle modifications, such as dietary modification and exercise. However, large randomized control studies are needed to provide the required evidence for this potential benefit. Hence, we used probiotic treatment compared with a placebo in a double-blind randomized control trial for a period of 6 months.
The study revealed reduction of BMI-standard deviation score after 6 months in both arms. However, it failed to show an added advantage of probiotics in obese children. Similarly, placebo arm demonstrated improvement in serum triglycerides, AST, ALT, AST/ALT ratio, and ALP without showing statistically significant benefit compared to the probiotics arm. Therefore, it is clear that lifestyle modifications play a role in improving the mentioned parameters. But insignificant improvement in probiotic arm could be due to probiotic strains used insufficient treatment duration or subtherapeutic dosage used despite the compliance being assured during the study. Hence, future studies should be designed with different probiotics strains, lengthier treatment plans, and higher probiotic dosages. It is noted that, Alisi et al. [38] showed supplement constituted an almost identical probiotics strains (VSL#3) to our study significantly improved NAFLD in children in comparatively much higher dosages (1 sachet per day for children under 10 years of age and 2 sachets per day for others).
Also, several important metabolic parameters including fasting blood sugar, oral glucose tolerance test, fasting insulin together with post prandial insulin and total cholesterol did not show significant improvement in both arms. This finding highlights the importance of finding different therapeutic modalities to manage metabolic syndrome in children. Therefore, further research should be focused on therapeutics options that could improve glucose and insulin metabolism in obese children.
In contradictory to metabolic parameters, USS grading of liver showed marked improvement in probiotic arm especially in subject categorized as USS stage I–II or II at baseline while the control group failed to show such improvement. However, sample size was small in the concerned group indicating that power should be increased to draw definitive conclusion. Also, it was noted that both arms are beneficial in down staging ultrasound grade I fatty liver to normal USS.
Transient elastography performed in selected group of subjects did not show significant improvement in both arms, again most probably due to the small sample size. Also, it may be due to its limited role in assessing NAFLD/NASH in obese subjects.
However, no adverse effect was reported during the study period, which is promising in view of consideration of nonharmful therapeutic options in managing obese children with NAFLD/NASH.
In conclusion, probiotic treatment for 6-month duration is not superior to conventional management (when compliance to lifestyle measures are satisfactory) of obesity-related NAFLD/NASH. However probiotic treatment seems to reduce the BMI and improved the fatty liver USS grade I–II/II.
Future studies should be designed with more power, different probiotic strains, dosages, and longer period of treatment. Also, improving glucose metabolism in obese children needs new therapeutic approaches since the current study failed to demonstrate significant improvement in glucose metabolism in both arms.
Supplementary materials
Supplementary materials 1-3 can be found via https://doi.org/10.3345/cep.2021.00787.
Amount of food portions to be taken from each food group for each age category
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
The study was supported by an educational grant by Probiotics International Ltd (Protein), UK.
Acknowledgements
We thank all children and their parents for participating in the study. We thank Ms. SMTH Senevirathna, Ms. TKG Rathnayaka, Mr. SDD Dissanayaka, Mr. DRS Jayasinghe from Department of Paediatrics, Faculty of Medicine, University of Colombo, the phlebotomist of the Professorial Paediatric Unit at Lady Ridgeway Hospital, Colombo for their enormous support in processing and analysis of the samples.