Efficacy of probiotics for managing infantile colic due to their anti-inflammatory properties: a meta-analysis and systematic review
Article information
Abstract
Background
Infantile colic (IC) is excessive crying in otherwise healthy children. Despite vast research efforts, its etiology remains unknown.
Purpose
Most treatments for IC carry various side effects. The collection of evidence may inform researchers of new strategies for the management and treatment of IC as well as new clues for understanding its pathogenesis. This review and meta-analysis aimed to evaluate the efficacy and possible mechanisms of probiotics for mananaging IC.
Methods
Ten papers met the study inclusion and exclusion criteria, and the meta-analysis was conducted using Review Manager (RevMan) software and a random-effects model.
Results
This meta-analysis revealed that probiotics are effective for treating infantile colic, while the review showed that this efficacy may be due to their anti-inflammatory effects.
Conclusion
Probiotics may be an important treatment option for managing infantile colic due to their anti-inflammatory properties.
Key message
Question: Do probiotics reduce colic symptoms?
Finding: Probiotics reduced colic symptoms in colicky infants probably due to the anti-inflammatory properties.
Meaning: Probiotics may be an effective and less noxious way to manage infantile colic.
Graphical abstract
Introduction
Infantile colic (IC) is a common problem in infancy that induces excessive crying in infants during the first few months of life [1,2]. IC is characterized by excessive crying at least 3 hours a day on at least 3 days a week for the duration of about 3 weeks leading to parental exhaustion, stress, anxiety, and depression [3,4]. The symptoms of IC peak at 6 weeks and disappears in the 12 weeks of age [5].
Previously studies have indicated IC follows a circadian rhythm of pain starting in the evening [6] also the prevalence of colic follow an equal pattern between male and female infants [7]. The pathogenesis of IC is not well understood. This condition is described as a multifactorial syndrome [8]. There are some options included in the aetiology of IC such as food allergies to milk proteins, an excessive amount of gas in the intestinal tract and intestinal hormones abnormalities. Recently some studies have declared the role of dysbiosis of microbiota, maternal smoking, and hormone alterations as well as gastrointestinal inflammation (GII) for the development of colic [9-11]. Some studies also indicated a correlation between parental issues and IC such as postpartum depression, parental anxiety, stressful pregnancies, dissatisfaction with the sexual relationship, negative experiences during childbirth, poor parental skills, and dysregulation of gut-brain axis [7]. the global incidence of IC is about 20% [12]. This condition makes one in 6 families to consult a healthcare system about the problem of excessive crying [13]. Still, there is not any kind of approved medications to fix this problem for instance simethicone failed to show superior characteristics over placebo [14,15]. Other studies also have shown promising effects of Dicyclomine hydrochloride in the treatment of IC but the incidence of side effects caused it to be contraindicated in infants [16].
Very low-quality shreds of evidence describe some other options for the treatment of this condition such as Cimetropium bromide, or Herbal agents But due to the lake of powerful evidence their administration for IC is not approved comprehensively [17]. Another option for the treatment of IC may be probiotic supplementation. Probiotics are nonpathogenic live strains of bacteria that when administrated in adequate amounts exert beneficial effects on the host. Prebiotics are substances that promote the growth of probiotic bacteria playing a vital role in the modulation of the gut microbiome a synbiotic is a combination of synergistically acting probiotics and prebiotics [18,19]. probiotics consist mostly strains of the genera Bifidobacterium and Lactobacillus, additionally, strains of Bacillus, Pediococcus and some yeasts can be introduced as suitable candidates. These strains together play an important role in the protection of the host against pathogen microorganisms and also host's immune system strengthens additionally in the maintenance of the intestinal microbial balance as well as anti-inflammatory responses [20,21].
Studies also revealed that low-grade systemic inflammation may be another important etiological factor for IC which is covered by other factors leading to being neglected [11].
Previous studies declared about increased levels of inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α) as well as interleukin-6 (IL-6) in the blood of patients experiencing functional gastrointestinal disorders when compared to control group [22,23].
Darkoh et al. [24] in a study showed significantly higher levels of inflammatory cytokines such as Interferon-gamma (IFN-γ), IL-1β, and TNF-α in the people experiencing gastrointestinal disorders such as irritable bowel syndrome moreover the concentration of chemokines such as monocytes chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), and chemokine (C-X-C motif) ligand 16 was significantly higher in the patients experiencing gastrointestinal disorders. They also revealed that anti-inflammatory cytokines found to be lower in the patients experiencing gastrointestinal disorders all of them in comparison to control group.
Calprotectin a marker of colonic inflammation is increased in infants experiencing colic [25]. Another study revealed that forkhead box P3 (FOXP3) a member of the forkhead transcription factor family is decreased in colicky infants that are another indicator of inflammation [26,27].
Based on the role of inflammation in functional gastrointestinal disorders especially colic it may be hypnotized that reducing inflammation would be a treatment strategy for IC. The present study aims to evaluate the role of probiotics as a treatment option for IC with a brief description of anti-inflammatory roles.
Methods
1. Inclusion criteria
The studies were included if they were qualified based on the following criteria: (1) expressed a randomized controlled trial (RCT), (2) consisted of a control group and a clinical cohort and the clinical cohort was based on the consumption of probiotics, (3) the scales were described as mean±standard deviation (SD), (4) the time course for the report of crying time was minute/day, (5) accessibility to the full text, and (6) published in a scientific journal.
2. Exclusion criteria
The studies were excluded if they: (1) did not qualify based on our inclusion criteria; (2) the report of crying time was not in minute/day; (3) the full text of paper was not accessible or the paper was not published in a scientific journal; (4) results were not expressed as means±SDs.
3. Search strategy
This review includes data about the effectiveness of probiotics for the management of IC as well as the role of inflammation in IC and probiotics with anti-inflammatory properties as a therapeutic option for treatment and control of IC until June 10, 2020.
Scientific databases such as Web of Science, PubMed, Science Direct, Scopus, and Google Scholar were used.
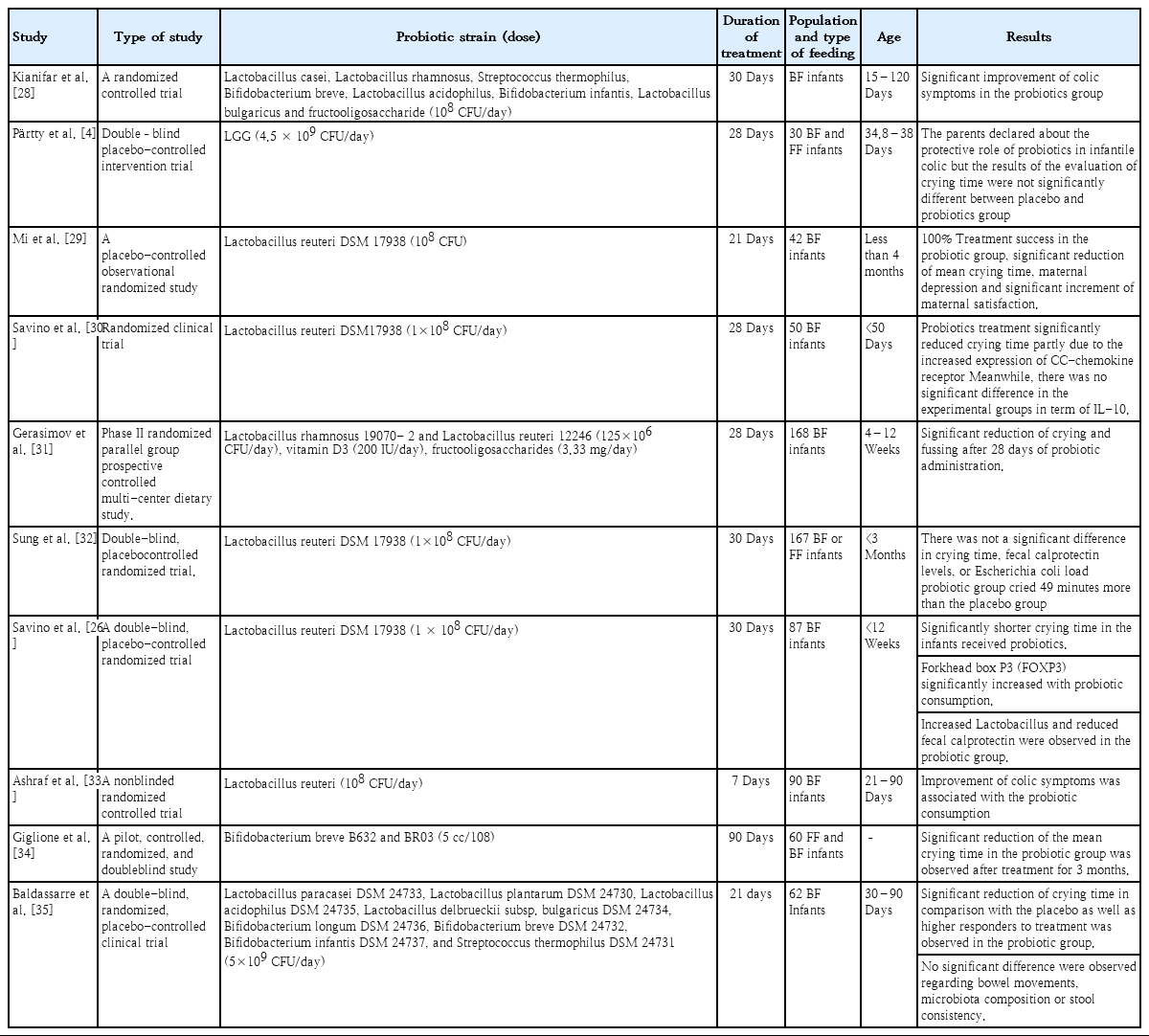
Keywords such as infantile colic, probiotic strains, anti-inflammation, inflammation, cytokines, gastrointestinal inflammation, TNF-α, IFN-γ, IL-10, IL-8, dysbiosis, gut microbiome, fecal calprotectin (FC), breast-fed infants, formula-fed infants, colic symptoms, and daily crying as well as their combinations were used. Finally, about 167 articles from 1954–2020 were found that after qualification based on the inclusion criteria and exclusion criteria 10 papers were included in our meta-analysis. the data for meta-analysis were extracted using a form collection of the dose regimen outcome and the other relative data (Table 1). The articles whose language was not English and the full text was not accessible were excluded.
4. Statistical analysis
The analysis was performed using the Review Manager (RevMan) Version 5.4. (The Cochrane Collaboration, 2020. Nordic Cochrane Center, Copenhagen, Denmark). the study outcome variables were described as standard mean differences (SMD) and a random effect based on the heterogeneity between the studies was chosen. we also used I2 for evaluation of heterogeneity between the studies If the I2 was ≥50%, the result was considered to exhibit a significant heterogeneity, and the specific model for evaluation of parameters was the random effect model. The risk of bias for each of the RCTs was evaluated using the Cochrane “risk of risk” assessment tool based on the author's judgments.
Results
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram shows the process in which the studies were included for our meta-analysis a total of 126 studies were screened and after qualification based on the inclusion and exclusion criteria, a total of 10 papers (including 367 intervention cases and 345 controls, Table 1) were entered the meta-analysis (Fig. 1).
1. Quality evaluation
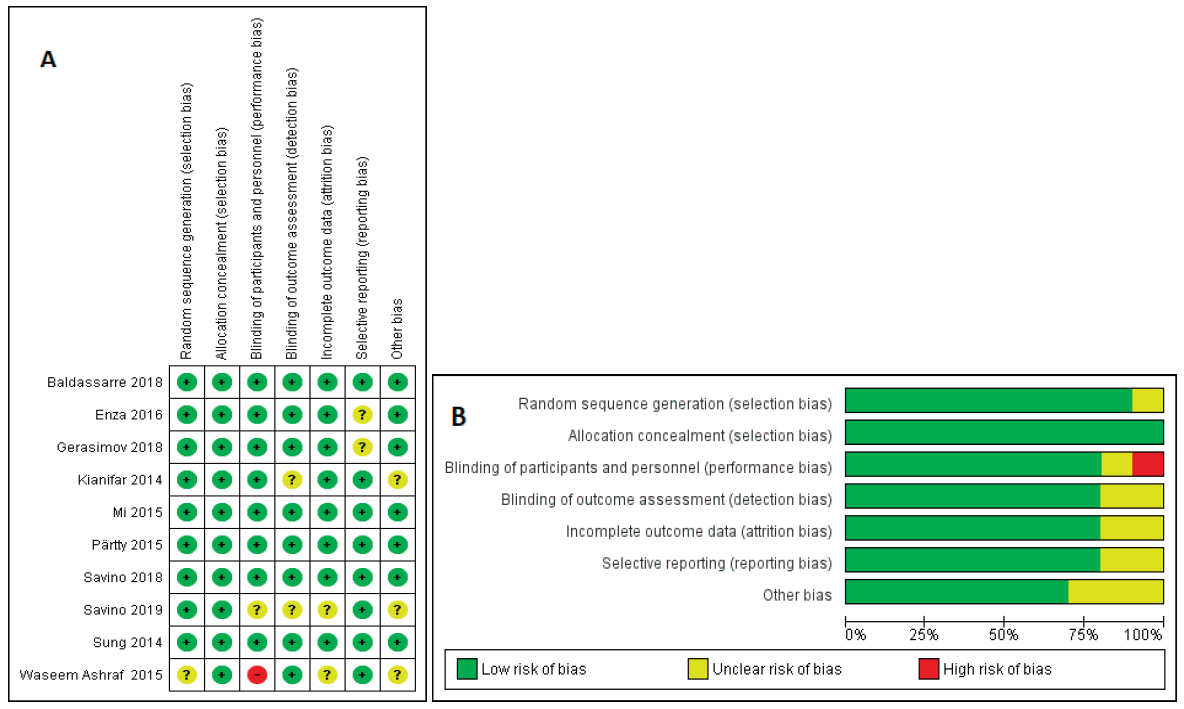
The risk of bias among the studies in the meta-analysis using the Cochrane “risk of risk” assessment tool was evaluated based on the author's judgment and the results are presented in Fig. 2. As it is obvious the risk of bias among the studies was relatively low. all the studies used probiotic as an intervention and placebo as the control group. In one of the studies, the intervention was not blinded. All other studies were randomized and the intervention was blinded properly.
2. Efficacy of probiotics in IC
As it is shown in Fig. 3, the meta-analysis revealed that probiotics effectively reduce the main symptom of IC that is excessive crying (SMD=-1.98; 95% confidence interval [CI], -2.74 to -1.22).
Discussion
In the present study, we investigated the efficacy of probiotics in the treatment of IC our results showed that probiotics can effectively reduce excessive crying in colicky infants the results of our meta-analysis showed a significant effect of probiotics for the treatment and management of IC (SMD=-1.98; 95% CI, -2.74 to -1.22).
Evaluation of the risk of bias between the studies showed quite a low risk of bias between the included studies. A random model was chosen for the assessment that’s why the included studies were different in some factors such as age, duration of treatment, and the dose of intervention. It would be more reliable to use a random effect in such a situation [36].
Skonieczna-Żydecka et al. [37] in a meta-analysis showed the efficacy of probiotics for the management of IC (MD=-2.012; 95% CI, -2.763 to -1.261).
Another study conducted to evaluate the efficacy of probiotics for reduction of crying time showed that after 4 weeks of treatment probiotics significantly reduced the crying time (weighted mean difference [WMD]=-56.32; 95% CI, -89.49 to -23.16; P=0.001) [38]. Sung et al. [39] in a study based on the meta-analysis declared that probiotics effectively reduce the crying time in colicky infants (MD=-67.72; 95% CI, -99.79 to -35.64).
A meta-analysis composed of 32 RCTs and 2,242 patients showed a superior effect for probiotic Lactobacillus reuteri DSM 17938 when compared to other treatments (WMD, -51.3; 95% CI, -72.2 to -30.5]; P=0.0001) [40]. The results of these studies are in concordance with our studies.
Inflammation is a physiologic response protecting cells from various injuries or infections that terminates automatically using endogenous or exogenous anti-inflammatory agents leading to hemostasis in various tissues and organs but when this regulation disrupts the result is uncontrolled inflammation is associated with pathologic conditions especially in the gastrointestinal tract [41,42]. Inflammation remarked by increased FC is associated with IC meanwhile decreased crying is observed in infants with lower levels of FC [25,43,44]. But some studies also failed to show a statistically significant correlation between fussing, crying, and FC [4,45].
Researches declared that FC may be influenced through the various source of infantile feedings such as formula or breastfeeding. Formula-fed infants usually indicate lower levels of FC but FC is increased in breast-fed infants, after all, some studies indicated that regardless of the source of feeding increased FC is associated with IC [25,26,46]. FC is increased in various inflammatory diseases such as inflammatory bowel diseases and can be used for assessment of the inflammatory situation in these patients also some other studies expressed that FC is an indication of granulocyte migration through the gut wall [47-49]. Increased intestinal permeability is found in various inflammatory situations in the gastrointestinal tract and the studies have observed a significant correlation between Calprotectin and intestinal permeability as a consequence of transepithelial granulocytes migration and inflammation [50]. There are some studies about absorption of macromolecules during IC indicating increased intestinal permeability during this situation these may reflect inflammatory situations during colic [51-54].
IC also induces alterations in other components of immune system profile such as MIP-1β, MCP-1/CCL2, and IL-8 that were significantly higher in the serum of infants experiencing colic additionally the serum concentration of TNF-α looks to be higher in the colicky infants notably indicating a systemic inflammation involved in it [11,55-57].
As we all know immune system composed of 2 major components including innate and adaptive immune systems that probiotics can exert various effects in both of them, this may inform about the role of probiotics in inflammation that is shown in previous studies too [58-61].
Probiotics are well known for their role in inflammation partly due to their effect to reduce the FC and/or fecal lactoferrin indicated by different studies [26,62,63].
Santas et al. [64] showed that treatment with probiotics Pediococcus pentosaceus CECT 8330 and Bifidobacterium longum CECT 7894 is associated with increased IL-10 production along with the significant reduction of crying time in infants with excessive crying. As it is obvious we all know the anti-inflammatory roles of IL-10 in the pathogenesis of various diseases [65].
Administration of L. reuteri DSM 17938 significantly increased the values of Foxp3+ T cells in the ileum besides this probiotic decreased the percentage of these cells in the mesenteric lymph nodes in a rat model of Necrotizing enterocolitis [66].
Additionally, this probiotic strain also reduced the ratio of retinoid-related orphan receptor-γ/Foxp3 messenger RNA levels due to significant increment of Foxp3 mRNA levels leading to the significant reduction of crying time in colicky infants as a result of modulation of T cells functions [26].
Foxp3+ regulatory T cells (Tregs) are known to be associated with maintenance of gut homeostasis, control of inflammation, inducing tolerance and also anti-inflammatory properties [67,68]. These pieces of evidence also highlight the immunomodulatory/anti-inflammatory roles of probiotics, especially in IC.
L. reuteri DSM17938 administration at a dose of 108 colony-forming unit in 28 days significantly increased the CC-chemokine receptor 7 in infants whose crying time was reduced. CC-chemokine receptor 7 is a homeostatic chemokine receptor that modulates leukocyte trafficking, induces and also silences inflammation between the peripheral tissues and the lymphoid tissue [30,69].
The efficacy of probiotics for management of IC is also summarized in the Table 2.
Administration of probiotics also have shown anti-inflammatory effects in other models of inflammatory gastrointestinal disorders for instance: Liu et al. [66] performed a study on probiotics all of which revealed anti-inflammatory effects through various approaches for instance: L. reuteri strains (ATCC PTA 4659, 5289, and 6475) significantly reduce Lipopolysaccharides (LPS) induced IL-8 production in IPEC-J2 (intestinal porcine enterocytes) cell line (obtained from the jejunal epithelium of a neonatal piglet). Incubation of rat intestinal epithelial cell line iec-6 (intestinal epithelioid cell) (obtained from the rat crypt) with LPS significantly increased cytokine-induced neutrophil chemoattractant-1 (CINC-1) (equivalent to IL-8) but L. reuteri strain 4659 significantly reduced CINC-1 as a marker of anti-inflammatory effects of probiotics [70]. Probiotics also exert anti-inflammatory effects in vivo. Keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO) (Cxcl1 or CINC-1) (an inflammatory indicator with similar function to human IL-8) in ileal tissues lysates were significantly increased after LPS administration in newborn rat pups but L. reuteri strains ATCC PTA4659, DSM17938, ATCC PTA 6475, and ATCC PTA 5289 significantly reduced this ratio [70]. The ratio of KC/GRO also expresses an activated Nuclear factor kappa-B (NF-κB) pathway which promotes the production of inflammatory cytokines. So reduced KC/GRO is proof for anti-inflammatory effects of probiotics [71]. Further investigations on the anti-inflammatory effects of probiotics result in revealing the role of probiotics on the expression of type 1 and 2 T helper (Th1 and Th2) cytokines in the rat intestine. Cytokines associated with Th1 include: IFN-γ, TNF-α, and IL-1β meanwhile Th2-type cytokines include IL-4, IL-5, and IL-13 in the rat intestine. L. reuteri strains (ATCC PTA4659, DSM17938, ATCC PTA 6475, and ATCC PTA 5289) significantly reduced the LPS-induced mRNA overexpression of IFN-γ. The levels of TNF-α or IL-1β were significantly decreased by various strains of probiotics. L. reuteri ATCC PTA 4659 and 5289 significantly decreased LPS-induced TNF-α production additionally L. reuteri DSM 17938 and ATCC PTA 6475 induce significant decrement of IL-1β. It was also observed that L. reuteri strain DSM 17938 or ATCC PTA 4659 significantly downregulates the mRNA level of IL-13 in the intestine when compared to the group treated with LPS. Inhibition of IL-4 and IL-5 overproduction due to LPS treatment was observed by L. reuteri strain DSM 17938. All of these cytokines are involved in the pathogenesis of various GIIs [70,72-74]. The results suggested above is indications of in vivo and in vitro anti-inflammatory effects of probiotics too.
Increased TNF-α is associated with IC as described previously [11] meanwhile administration of specific L. reuteri strains such as ATCC PTA 6475 and ATCC PTA 5289 can decrease TNF-α production in the monocytoid THP-1 cells [61]. Lin et al. [21] in their study showed that L. reuteri strain ATCC PTA 6475 potentially decrease the LPS-induced increment of TNF-α in Monocytes and primary monocyte-derived macrophages isolated from children experiencing Crohn disease. The chemokine MCP-1/CCL2 was also reduced in macrophages of children received this strain of probiotic who were experiencing alleviation.
Scully et al. [75] in a study showed that Bifidobacterium infantis 35624 possess the ability to significantly reduce Peyer’s patch macrophage inflammatory protein 1α (MIP-1α) and MIP-1β secretion induced by salmonella infection that is partly due to increased Peyer’s patch CD4+CD25+ regulatory T cells.
Another study showed that the supplementation of ageing mice with L. acidophilus DDS-1 modulated the gut microbiome community and significantly reduced the serum levels of MIP-1β when compared to the control group [76]. As described previously increased MIP-1β in serum is associated with IC.
Two strains of probiotics L. reuteri DSM 17938 and ATCC PTA 4659 were investigated for their immunomodulatory effects in LPS-induced ileitis in rats, the results of this study showed anti-inflammatory effects of probiotics evidenced by significant downregulation of TNF-α, IL-6, toll-like receptor-4 (TLR-4), and NF-κB as well as significant up-regulation of IL-10. As described previously TNF-α, IL-6, TLR-4, and NF-κB are markers of the inflammatory situation and IL-10 is a potent anti-inflammatory cytokine which all of them announce probiotics as anti-inflammatory and immunomodulatory agents [77]. Probiotics especially L. reuteri has shown the potency against proinflammatory cytokine TNF-α production in mice experiencing colitis [78] leading to reduced IL-8 production [79].
Another study showed that IL-10 deficient mice exhibited lower levels of colonic L. sp. And normalizing the levels of this probiotic reduce colonic mucosal adherent leading to alleviation of statues in colitis [80].
The gut microbiome is one important factor for protecting the gastrointestinal tract from various pathogenic conditions, the imbalanced gut microbiome is associated with various pathogenic situations especially colic [81,82].
Some studies also declared that dysbiosis of the gut microbiome is associated with inflammation which is observed in IC too [11,25]. The gut microbiome is known to produce some products such as short-chain fatty acids that perform anti-inflammatory and immunomodulatory effects. Microbiome community in the gut also exert immunomodulatory functions not only in the gastrointestinal tract but also in wider aspects such as systemic immune disorders [83-85]. So balanced gut microbiome is an important issue in the pathogenesis of IC which should be specially taken into consideration.
There is a lot of studies about protecting the roles of probiotics against gut microbiome dysbiosis [86-88]. This also highlights the anti-inflammatory roles of probiotics in the gastrointestinal tract.
In conclusion, some specific strains of probiotics exert desirable efficacy for treatment of IC through multiple therapeutic ways including anti-inflammatory roles. Preservation of gut microbiome homeostasis is another therapeutic role accomplished by probiotics. The complicated base of IC with limitations in the treatment and diagnosis may introduce specific strains of probiotics as a new therapeutic strategy for the management of this burden.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
Hereby, it would give us great pleasure to thank all researches whose articles were used in this study!