Updates in neonatal resuscitation: routine use of laryngeal masks as an alternative to face masks
Article information
Abstract
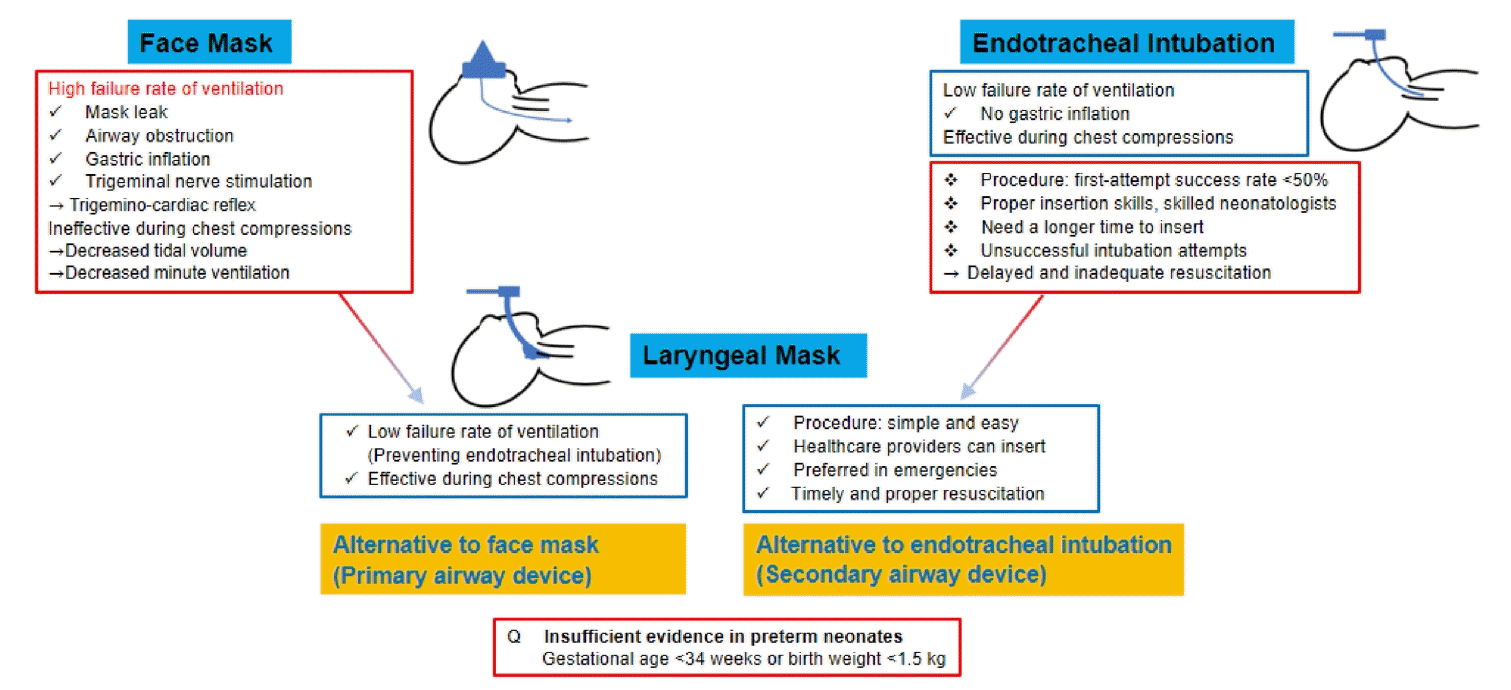
Although positive-pressure ventilation (PPV) has traditionally been performed using a face mask in neonatal resuscitation, face mask ventilation for delivering PPV has a high failure rate due to mask leaks, airway obstruction, or gastric inflation. Furthermore, face mask ventilation is compromised during chest compressions. Endotracheal intubation in neonates requires a high skill level, with a first-attempt success rate of <50%. Laryngeal masks can transfer positive pressure more effectively even during chest compressions, resulting in a lower PPV failure rate compared to that of face masks in neonatal resuscitation. In addition, inserting a laryngeal mask is easier and more accessible than endotracheal intubation, and mortality rates do not differ between the 2 methods. Therefore, in neonatal resuscitation, laryngeal masks are recommended in infants with gestational age >34 weeks and/or with a birth weight >2 kg, in cases of unsuccessful face mask ventilation (as a primary airway device) or endotracheal intubation (as a secondary airway device, alternative airway). In other words, laryngeal masks are recommended when endotracheal intubation fails as well as when PPV cannot be achieved. Although laryngeal masks are commonly used in anesthetized pediatric patients, they are infrequently used in neonatal resuscitation due to limited experience, a preference for endotracheal tubes, or a lack of awareness among the healthcare providers. Thus, healthcare providers must be aware of the usefulness of laryngeal masks in depressed neonates requiring PPV or endotracheal intubation, which can promptly resuscitate these infants and improve their outcomes, resulting in decreased morbidity and mortality rates.
Key message
In neonatal resuscitation:
· Laryngeal masks are recommended when endotracheal intu-bation or positive-pressure ventilation fails.
· Laryngeal masks are useful even during chest compressions.
· Laryngeal masks aid neonates >34 weeks’ gestation and/or with a birth weight >2 kg.
· Main usage barriers include limited experience (81%), pre-ference for endotracheal tubes (57%), and lack of awareness (56%).
· Second-generation laryngeal masks have a built-in esophageal drainage tube that prevents regurgitation into the glottis, and an orogastric tube can be inserted within the esophageal drainage tube to protect against gastric inflation.
Introduction
Ventilation of the lung is the most important and effective step in neonatal resuscitation [1]. Positive-pressure ventilation (PPV) is crucial for neonatal resuscitation, as cardiac failure occurs after respiratory failure in neonates. Therefore, ineffective ventilation can lead to cardiac failure, requiring intubation, chest compression, or epinephrine administration. PPV in neonates is traditionally achieved using face mask ventilation [2]. However, a high risk of failure is associated with PPV delivery owing to factors, such as mask leakage, airway obstruction, gastric inflation, or trigeminal nerve stimulation causing the trigemino-cardiac reflex [3]. Furthermore, face mask ventilation becomes compromised during synchronized chest compressions due to increased mask leakage, resulting in decreased tidal volume and minute ventilation [4,5]. Endotracheal intubation has a lower failure rate than face mask ventilation in delivering PPV without gastric inflation, thereby making it more effective during chest compressions. However, endotracheal intubation in neonates requires a substantial amount of simulated training and experience to acquire proper insertion skills, which need a longer time than the insertion of a laryngeal mask. Studies using laryngeal masks in anesthetized pediatric patients beyond infancy have shown that the laryngeal mask is superior to face mask ventilation and similar to endotracheal intubation [6]. However, studies are lacking on the use of laryngeal masks in neonatal resuscitation, especially in preterm infants. Therefore, this review aimed to compare the effectiveness of laryngeal mask versus face mask or endotracheal intubation for PPV during neonatal resuscitation. Furthermore, we compared the different types of laryngeal masks used in neonatal resuscitation.
Introduction of laryngeal masks to initial PPV in neonatal resuscitation
Laryngeal masks have been used in neonatal resuscitation for several years. The American Heart Association and American Academy of Pediatrics introduced laryngeal masks as an alternative to endotracheal tubes (as a secondary airway device) after intubation failure in the neonatal resuscitation program (NRP). However, they did not recommend the use of laryngeal masks as an alternative to face mask ventilation (as a primary airway device). As laryngeal masks are less invasive and relatively easy to insert without laryngoscopy, they have been studied as an alternative to face mask ventilation during the initial stages of neonatal resuscitation. In 2020, the NRP recommended laryngeal masks as an alternative to face masks as the primary airway device or endotracheal intubation as the secondary airway device. Laryngeal mask insertion was included in Lesson 4 on PPV (NRP Essentials) in the 8th edition of the NRP [7], a change from Lesson 5 on endotracheal intubation in the 7th edition of the NRP [8]. The International Liaison Committee on Resuscitation recently suggested the use of laryngeal masks in infants with a gestational age >34 weeks and/or a birth weight >2 kg in cases of face mask ventilation or endotracheal intubation failure.
Laryngeal masks versus face mask ventilation in neonatal resuscitation
Six ventilation-corrective steps were performed to correct common problems associated with face mask ventilation during neonatal resuscitation. The 6 ventilation-corrective steps are collectively known as “MR. SOPA” and are performed in the following order: mask adjustment, reposition airway, suction mouth and nose, open mouth, pressure increase, and airway alternative; these steps improve the effectiveness of face mask ventilation and prevent failures in face mask ventilation [9]. The laryngeal mask is less likely to leak or cause an airway obstruction, and it can more effectively transfer positive pressure during chest compressions than a face mask. A systemic review by Qureshi and Kumar [10] of 5 studies involving 661 infants reported that PPV and resuscitation duration were shorter when a laryngeal mask was used for ventilation compared to a facemask.Additionally,the risk of PPV failure and need for endotracheal intubation were lower when a laryngeal mask was used for ventilation compared to a face mask. A recent systemic review by Diggikar et al. [11] of 6 studies including 1,853 infants with a gestational age of ≥34 weeks and birth weight of ≥1.5 kg or ≥2 kg (946 in the laryngeal mask group vs. 907 in the face mask group) reported that the risk of PPV failure and the need for endotracheal intubation were lower in the laryngeal mask group than that in the face mask group. Furthermore, time to recover spontaneous breathing and duration of ventilation were shorter in the laryngeal mask group than those in the face mask group, while the 2 groups had similar rates of mortality and moderate-to-severe hypoxic-ischemic encephalopathy [11]. A systemic review of 6 randomized controlled trials including 1,823 infants with a gestational age of ≥34 weeks reported that laryngeal masks are less likely to cause PPV failure and require endotracheal intubation than face masks [12]. Furthermore, laryngeal mask usage more quickly achieved a heart rate >100 beats per minute and decreased the PPV duration compared to face mask usage [12]. A randomized controlled trial in Uganda of 1,154 infants with a gestational age of ≥34 weeks or a birth weight of ≥2 kg (563 in the laryngeal mask group vs. 591 in the face mask group) reported that laryngeal masks were safely handled by midwives [13]. However, the risks of death within 7 days and of moderate-to-severe hypoxic-ischemic encephalopathy were not decreased when a laryngeal mask was used for ventilation. Table 1 describes studies comparing laryngeal and face masks in neonates within the last 10 years; older studies were excluded due to the rapid advancement of neonatal resuscitation in recent years.
Laryngeal masks versus endotracheal intubation in neonatal resuscitation
Endotracheal intubation requires proper insertion skills and laryngoscopy to confirm passage of the endotracheal tube through the vocal cords. However, laryngeal mask insertion is a relatively simple procedure. Endotracheal intubation requires neonatal resuscitation specialists, such as skilled neonatologists; however, healthcare providers competent in airway management can insert a laryngeal mask. According to a study by the National Emergency Airway Registry for Neonates, the first-attempt success rate of endotracheal intubation was <50%: 49% in the neonatal intensive care unit and 46% in the delivery room [14]. Therefore, laryngeal mask insertion may be preferred in emergencies, such as neonatal resuscitation at birth [15]. Additionally, some experts are concerned about delayed and inadequate resuscitation as a result of unsuccessful intubation attempts considering a neonatal intubation first-attempt success rate of <50% and the risk of severe desaturation [16].
The mean laryngeal mask insertion time was within 10 s in neonates with a gestational age of ≥35 weeks or a birth weight of ≥2.5 kg [17]. However, according to a study in 2022, it took NRP providers 36 s to insert a laryngeal mask in a manikin versus 32 s to insert an endotracheal tube in the same manikin [18]. The NRP providers were less confident about laryngeal mask insertion than endotracheal intubation. A systematic review of 3 studies including 158 infants reported no significant differences in the insertion time or success rate between the laryngeal mask and endotracheal intubation [10]. Furthermore, no significant intergroup differences were noted in the mortality or hypoxic-ischemic encephalopathy rate. In a systematic review of 3 studies, Diggikar et al. [11] reported no significant differences in the rate of unsuccessful insertion, orofacial soft tissue injury, and 5-min Apgar scores between laryngeal mask and endotracheal intubation in infants with a gestational age of≥34 weeks or a birth weight of ≥1.5 kg or 2 kg. Table 2 describes studies comparing laryngeal masks and endotracheal intubation conducted on neonates within the last 10 years; older studies were excluded due to the rapid advancement of neonatal resuscitation in recent years.
Laryngeal masks during chest compressions in neonatal resuscitation
Face mask ventilation is suboptimal for PPV in neonatal resuscitation, especially during chest compressions, which impedes adequate ventilation and delays the next steps of resuscitation, such as vascular access or epinephrine administration [19,20]. Face mask ventilation during chest compressions is associated with decreased tidal volume and minute ventilation due to increased mask leakage compared to PPV alone [5]. Evidence supporting the use of laryngeal masks during chest compressions in neonatal resuscitation is limited primarily due to lack of experience. According to animal models comparing ventilation with a laryngeal mask and endotracheal tube during chest compressions, the mean airway pressure and expired tidal volume did not differ between lambs ventilated with a laryngeal mask or an endotracheal tube [21]. The median time to achieve a return of spontaneous circulation was similar in the 2 groups [21], indicating that ventilation with a laryngeal mask was not inferior to ventilation with an endotracheal tube during chest compressions. In a manikin study comparing laryngeal mask and face mask ventilation during chest compressions, the peak inspiratory pressure was higher. The time taken to complete 30 cycles of 3 compressions and one ventilation was shorter in manikins ventilated with a laryngeal mask than that in those ventilated with a face mask [22].
Laryngeal masks in preterm infants in neonatal resuscitation
The smallest laryngeal mask is size 1, which is designed to be suitable for infants weighing 2–5 kg. Therefore, insufficient evidence exists supporting the use of laryngeal masks in preterm infants. A randomized controlled trial comparing laryngeal masks to face masks or endotracheal intubation included infants with a birth weight of >1.5 kg. However, the authors did not provide information on the weight or number of infants with a birth weight of 1.5–2 kg [23]. Another study comparing laryngeal and face masks excluded infants with a birth weight of <1.5 kg [24]. However, this study did not provide information on the mean or range of birth weight or number of enrolled infants with a birth weight of 1.5–2 kg. In a randomized controlled trial by Trevisanuto et al. comparing laryngeal and face masks, the former were used in 22 infants with a birth weight of 1.5–2 kg [25]. Laryngeal masks have been used to administer surfactant to preterm infants, such as those with a gestational age of 30 weeks and a birth weight of 1.36 kg [26], a gestational age of 31 weeks and a birth weight of 1.335 kg [27], and a gestational age of 32 weeks with a birth weight of 1.53 kg [28]. Trevisanuto et al. reported the use of a laryngeal mask in preterm infants with a gestational age as low as 28 weeks and a birth weight as low as 880 g [29]. According to a pilot trial investigating the use of a laryngeal mask for surfactant administration in 13 preterm infants with a gestational age between 31 and 34 weeks and a birth weight between 1.67 kg and 2.82 kg, surfactant administration via laryngeal mask was feasible and successful [30]. Furthermore, laryngeal masks were successfully used to administer surfactant to preterm infants with a birth weight of ≥1.2 kg [31]. However, the use of a laryngeal mask on a preterm infant with a birth weight of 670 g led to upper esophageal injury, which required extensive antibiotic treatment [32]. Therefore, care is advisable while using laryngeal masks on preterm infants with a gestational age of ≤34 weeks or a birth weight of ≤2 kg, especially ≤1.5 kg.
Barriers to laryngeal mask use in neonatal resuscitation
According to a survey conducted in the United States on the use of laryngeal masks in neonates, only 12% of respondents had ever placed a laryngeal mask in a live newborn, and the respondents expressed low confidence in their ability to properly place the laryngeal mask [33]. The most common barriers to laryngeal mask use in neonates were limited experience (81%), insufficient training (59%), preference for endotracheal tube (57%), and lack of awareness (56%) [33]. According to a nationwide survey of the use of laryngeal masks in Brazil [34], most respondents recognized the usefulness of laryngeal masks in neonatal resuscitation, and over half of them (64%) were reported to know a laryngeal mask insertion. However, less than half (41%) were trained to use laryngeal masks. Limited experience was the most common barrier to laryngeal mask use in neonatal resuscitation. In this survey, only 8% of respondents reported having placed a laryngeal mask in the delivery room [34]. Another barrier to the use of laryngeal mask during neonatal resuscitation in Brazil was their unavailability. According to another survey of experience with laryngeal masks and endotracheal intubation in neonates in the United Kingdom, 47% had been trained for the use of laryngeal masks but only 7% had actually used it in a newborn infant [35]. Even NRP providers did not perceive laryngeal mask insertion as being less challenging than endotracheal intubation [18]. Additionally, they were not proficient at performing laryngeal mask insertion. This is another barrier to laryngeal mask use in neonatal resuscitation. Most studies on the use of laryngeal masks in neonatal resuscitation excluded preterm infants with a gestational age <34 weeks or birth weight <2 kg, especially <1.5 kg. Therefore, insufficient evidence is available to support the use of laryngeal masks in this population, which possess a barrier to their adoption in neonatal resuscitation.
Evolution of various laryngeal masks
Second-generation laryngeal masks have a built-in esophageal drainage tube that prevents regurgitated fluid from spilling into the glottis (Fig. 1). Additionally, an orogastric tube can be inserted to protect against gastric inflation [36]. Furthermore, second-generation laryngeal masks have been developed to improve airway protection and increase airway seal pressure. The Laryngeal Mask airway ProSeal (LMA North America, San Diego, CA, USA) is a second-generation laryngeal mask designed for reusability. The Laryngeal Mask Airway Supreme (LMA Supreme, Teleflex, Athlone, Ireland) is a second-generation laryngeal mask with a curved rigid airway tube. This disposable device is currently available in 2 sizes (1 and 2) for pediatric use [37,38]. All of these products are easily accessible domestically.

Second-generation laryngeal mask. The second-generation laryngeal mask incorporates a built-in esophageal drainage tube that prevents regurgitated fluid from spilling into the glottis. An orogastric tube can be inserted within the esophageal drainage tube to protect against gastric inflation. Furthermore, the secondgeneration laryngeal mask improves airway protection and increases airway seal pressure.
Conclusion
The NRP and International Liaison Committee on Resuscitation recommend laryngeal masks as primary and secondary airway devices in neonates when face mask ventilation or endotracheal intubation attempts are unsuccessful. However, laryngeal masks are not frequently used in neonatal resuscitation because of limited experience, a preference for endotracheal tubes, or a lack of awareness among healthcare providers. Unsuccessful intubation attempts can impede timely and proper resuscitation, resulting in severe deterioration of neonates. Laryngeal mask insertion is easy and simple, making it a preferred choice in emergencies for promptly resuscitating neonates. Healthcare providers must be aware of the usefulness of laryngeal masks in depressed neonates requiring PPV or endotracheal intubation, which can improve the outcomes in such cases, as well as decrease morbidity and mortality rates. Insufficient evidences are currently available for the use of laryngeal masks in preterm infants, particularly those with a gestational age of ≤34 weeks or a birth weight of ≤2 kg, especially ≤1.5 kg. Therefore, further research is needed to determine the safety and efficacy of laryngeal masks specifically in this vulnerable population.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by the Inha University Research Grant.