Diabetic ketoacidosis in children induced by coronavirus disease 2019 (COVID-19) diabetic ketoacidosis post-COVID-19 in children
Article information
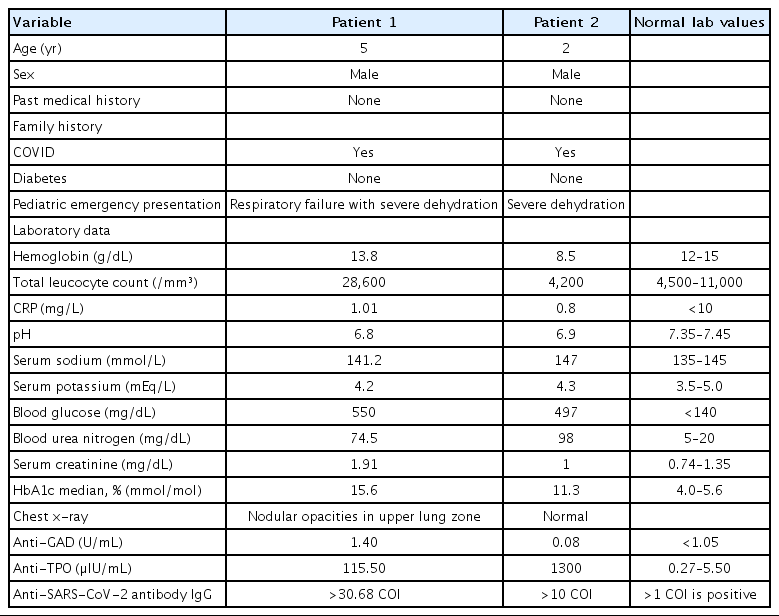
Two cases of diabetic ketoacidosis (DKA) were admitted with history of contact being coronavirus disease 2019 (COVID-19) positive a month prior to admission. Both cases had acute onset of polyphagia and polydipsia without any past history of diabetes or any other comorbidities. Both were anti-severe acute respiratory syndrome coronavirus-2 (anti-SARSCoV2) antibody positive. Table 1 summarises the clinical features and laboratory results of both patients. Our first case was a 5-year-old malnourished boy weighing 13 kg (10th centile as per Indian Academy of Paediatrics growth chart) admitted with severe dehydration and respiratory failure. The patient was ventilated for 1 day, he remained on continuous insulin infusion for 2 days then was switched to subcutaneous insulin regimen once the acidosis resolved. After obtaining adequate glucose control child was shifted to fast acting Lispro insulin (Humalog by Shaverix Impex India Private Limited) and long acting Glargine insulin (Sanofi Lantus Insulin, 1 by Tava Drugs India) and then discharged on stabilization. Our second case was a 2-year-old boy, weighing 12 kg (50th centile as per Indian Academy of Paediatrics growth charts) admitted with severe dehydration. He was rehydrated slowly, given continuous insulin infusion and thyroxine replacement for associated hypothyroidism. He responded to treatment and was discharged 7 days postadmission.
Diabetes and COVID-19 infection in adult and children
The COVID-19 and diabetes are known to be a deadly combination in adults, but literature on impact of COVID on diabetic children or vice versa are limited. Data in adult population suggests higher prevalence of severe COVID infection in diabetics [1,2]. Guan et al conducted a nation wise analysis of COVID-19 patients to find out the most common comorbidity and its impact [3]. Patients with diabetes mellitus having high inflammatory markers and severe insulin resistance had worse prognosis. A systematic review of 110 adult patients with DKA or hyperosmolar hyperglycemic syndrome showed only 77% of them had pre-existing diabetes. Remaining had presented with metabolic complications for the first time post-COVID-19 infection [4]. This finding is similar to our case series where children were diagnosed with diabetes mellitus after they had presented with complications.
DKA as the first presenting sign of COVID-19
DKA arises from a relative insulin deficiency. Early reports indicate that among patients with pre-existing diabetes, DKA may be a common complication of severe COVID-19 and a poor prognostic sign. Children presenting with DKA as the first presenting sign of COVID-19 are rare. The first surge of type 2 diabetes with DKA in children was noticed by Chao et al. [5] in March 2020. They had stressed upon the urgency of evaluation of any child presenting with symptoms of diabetes to prevent DKA in them. COVID-19 pandemic had brought the world to a lockdown and many known patients of diabetes were not in regular follow-up of pediatricians. A recent multicentric study from United Kingdom has reported an increase in new-onset type 1 diabetes mellitus post-COVID-19 pandemic [6].
Association between the COVID-19 infection diabetes and immune dysregulation
Adult reports suggest hyperglycaemia is due to SARSCoV2 infecting insulin secreting cells triggering inflammation, leading to pleiotropic alterations in glucose metabolism [7]. It is difficult to interpret whether hyperglycaemia in COVID-19 is a cause, or, a consequence of severe disease. Besides hyperglycaemia viral infection is known to cause immune dysregulation. Immune dysregulation is any breakdown or impaired alteration in molecular control of immune system processes. “Middle East respiratory syndrome” coronavirus and HINI influenza pandemics are examples of it [8]. Viruses like Epstein Barr virus, hepatitis C virus, and human herpesvirus 6, trigger immune responses leading to rashes and various autoimmune disease. Common cold or influenza virus too can trigger abnormal cytokine response leading to immune dysregulation [9]. Until now there has not been a single case report of COVID-19 triggering both immune dysregulation involving more than one organ system.
Both cases had positive anti-SARS-CoV-2 antibodies IgG on admission nearly a month after positive contact. By the time, they had presented they had become COVID-19 negative but developed complications due to COVID-19. Glutamic acid decarboxylase positivity in first case indicates that child is developing antibodies against its own islet cells. The appearance of these autoantibodies signals an autoimmune pathogenesis of β-cell killing. Similarly, presence of thyroid peroxidase antibody in second indicates autoimmunity against thyroid. To the best of our knowledge, there have been isolated cases of DKA in children post-COVID-19 [10]. Ours is the first case series where both these patients developed severe metabolic complications along with other autoimmunity markers post-COVID-19.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.