A perspective on partially hydrolyzed protein infant formula in nonexclusively breastfed infants
Article information
Abstract
The World Health Organization recommends that infants should be exclusively breastfed for the first 6 months of life to provide optimal nutrition in this critical period of life. After this, infants should receive nutritionally adequate and safe complementary foods while breastfeeding continues for up to 2 years of age or beyond. For nonbreastfed infants, infant formula is an available option to provide the nutrition needed. Infant formula is usually prepared from industrially modified cow’s milk and processed to adjust for the nutritional needs of infants. However, cow’s milk is one of the most common causes of food allergy, affecting 2%–5% of all formula-fed infants during their first year of life. One strategy to prevent cow’s milk allergy in nonbreastfed infants is the use of partially hydrolyzed formula (pHF) in high-risk infants, which are infants born in families with atopic disease. However, based on an epidemiological study, approximately half of the infants who develop allergy are not part of the at-risk group. This is because the non-at-risk group is significantly larger than the at-risk group and the non-at-risk infants have approximately 15% risk of developing allergies. This study aimed to evaluate the effects of partially hydrolyzed whey formula (pHF-W) in nonbreastfed infants and determine whether pHF-W can prevent atopic disease in high-risk infants and can be used as routine starter formula regardless of the allergy risk status.
Introduction
Breastfeeding is the best way to provide optimal nutrition for the healthy growth and development of infants. Human breast milk contains nutrients such as carbohydrates, protein, fat, vitamins, and minerals. It also contains oligosaccharides, digestive enzymes, hormones, immune cells, and good bacteria, which are important to support the growth and development of infants [1]. The World Health Organization and the United Nations Children’s Fund recommend that infants should be exclusively breastfed for the first 6 months of life to provide optimal nutrition [2]. Breastfeeding should be continued up to the age of 2 years or beyond, while adequate and safe complementary foods are gradually introduced [2].
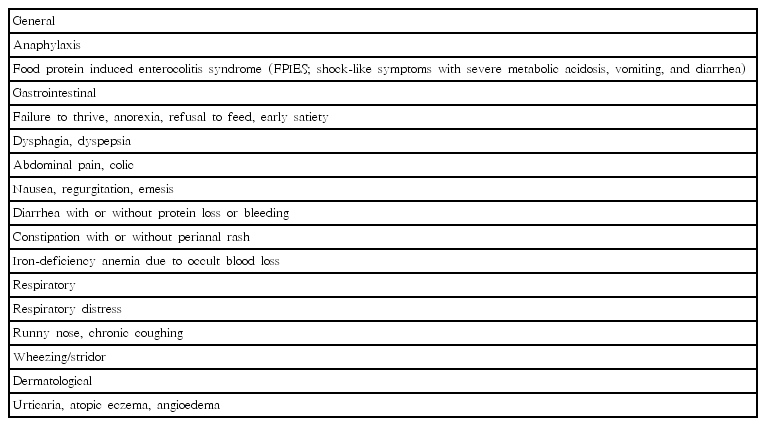
For nonbreastfed infants, infant formula is an available option to provide the needed nutrition. It is usually prepared from industrially modified cow’s milk and processed to adjust the nutritional content according to the needs of infants [3,4]. However, cow’s milk is one of the most common causes of food allergy, affecting 2%–5% of all formula-fed infants during their first year of life. Cow’s milk allergy (CMA) may involve various organ systems, induce a range of symptoms, and cause failure to thrive [5,6]. The most frequent symptoms and signs related to CMA are listed in Table 1.
Although CMA is common, the symptoms are nonspecific; therefore, CMA is not often diagnosed properly. This will cause a burden on the infant and the parents; therefore, prevention of CMA will be beneficial. One strategy to prevent CMA in nonbreastfed infants is the use of partially hydrolyzed formula (pHF). This type of formula was developed to reduce protein allergenicity through heat treatment and chemical and enzymatic hydrolysis, which results in a reduced molecular weight and peptide size. The molecular weight of pHFs is generally <5 kD (range, 3–10 kD), while whole cow’s milk-based formula contains peptides in the range of 14 kD (α-lactalbumin) to 67 kD (bovine serum albumin) [7]. Most guidelines recommend pHFs to prevent allergic disease, mainly atopic dermatitis (AD), and CMA in nonbreastfed infants at high risk [5]. However, based on an epidemiological study, approximately half of the infants who developed allergies are not part of the at-risk group [8]. This is because the non-at-risk group is significantly larger than the at-risk group and the non-at-risk infants have approximately 15% risk of developing allergies [9].
This study aimed to evaluate the effects of partially hydrolyzed whey formula (pHF-W) in nonbreastfed infants and determine whether pHF-W can prevent atopic disease in high-risk infants and can be used as routine formula regardless of the allergy risk status.
pHF for atopy prevention
Hydrolyzed formulas contain cow’s milk proteins (CMPs) that have been hydrolyzed to smaller peptides than those found in standard cow’s milk formula (CMF) [1]. The size of these peptides is determined by the degree of ultraheating and ultrafiltration as well as enzymatic hydrolysis, which will result in partial or extensive hydrolyzed formula [10]. The reduction of molecular weight and the peptide size of CMP potentially attenuate the allergenicity of the proteins and decrease potentially sensitizing allergenic determinants. The pHFs retain sufficient size of peptides and immunogenicity to induce oral tolerance with minimal sensitization [11].
The benefit on prevention of atopic disease by pHFs is affected by several factors, such as protein source (casein and whey), method of hydrolysis (type of enzyme[s] used, temperature, and pH), and degree of hydrolysis [12]. Therefore, the effect of pHFs should be evaluated for each hydrolyzed formula since not all pHFs are equal [13].
A meta-analysis from 2010 concluded that high-risk infants who were fed one 100% pHF-W showed a 55% reduction of AD incidence (summary relative risk [RR] estimate, 0.45; 95% confidence interval [CI], 0.40–0.70) and 44% reduced risk of any atopic manifestation (summary RR estimate, 0.56; 95% CI, 0.30–0.70) compared to those who were fed CMF [12].
Another updated meta-analysis reviewed the efficacy of the same specific 100% pHF-W and confirmed the efficacy of this formula by showing a statistically significant reduction of eczema according to the intention-to-treat analysis compared with CMF at different time intervals between 0 and 3 years (3 randomized controlled trials [RCTs]; n=1,000; RR, 0.82; 95% CI, 0.68–1.00) and 0 to 5–6 years (2 RCTs; n=938; RR, 0.83; 95% CI, 0.69–0.99) in infants at high risk. Per protocol analyses showed eczema risk reduction in favor of pHF compared with CMF at 0 to 3 years (3 RCTs; n=616, RR, 0.63; 95% CI, 0.48–0.82) and at 0 to 5–6 years (2 RCTs; n=500; RR, 0.72; 95% CI, 0.55–0.93) in this high-risk population [13]. The strongest evidence is provided by the independently funded “German Infant Nutritional Intervention” (GINI) study. The GINI study is a large, well-designed and conducted, randomized, double-blind (until 3 years of age) trial, with a 15-year follow-up. It compared the incidence of atopic disease in infants fed with standard CMF, pHF-W, and extensive whey or casein hydrolyzed protein formula. At 2 time intervals (0–10 years and 0–15 years), the results were of borderline statistical significance in favor of pHF. Follow-up studies until 15 years have shown a reduction of atopic dermatitis in the pHFs group compared with the standard CMF group [14].
In contrast to both meta-analyses, Boyle et al. [15], reviewed the literature, not limiting the included studies to pHF-W, and concluded that there was no evidence that partially or extensively hydrolyzed formula reduces the risk of allergy or autoimmune disease in infants. The odds ratio for eczema at age 0–4 months, compared with standard CMF, was 0.84 (95% CI, 0.67–1.07; I2=30%) for pHF and 0.55 (95% CI, 0.28–1.09; I2=74%) for extensively hydrolyzed formula. This finding was affected by a large study with a negative outcome on a different pHF-W than the one included in the abovementioned meta-analyses. A Cochrane review published in 2018 concluded that there is no evidence to support that short-term or prolonged feeding with a hydrolyzed formula prevents allergic disease compared with exclusive breastfeeding [16]. Very low-quality evidence indicates that short-term use of an extensively hydrolyzed formula may prevent infant CMA [16]. Twelve studies assessed the effects of prolonged infant feeding with a hydrolyzed formula compared with CMF, reporting no difference in all allergic disease in infants (RR, 0.88; 95% CI, 0.76–1.01) [16]. However, different hydrolysates were included in the analysis, and results of studies performed in preterm and term infants were combined [16]. As mentioned earlier, the outcome obtained with one hydrolyzed formula cannot be extrapolated to another hydrolyzed formula since they all differ in method of hydrolysis and thus in peptide structure and size [5]. Only one routine infant pHF-W has been granted a qualified health claim by the U.S. Food and Drug Administration for risk reduction of AD [17].
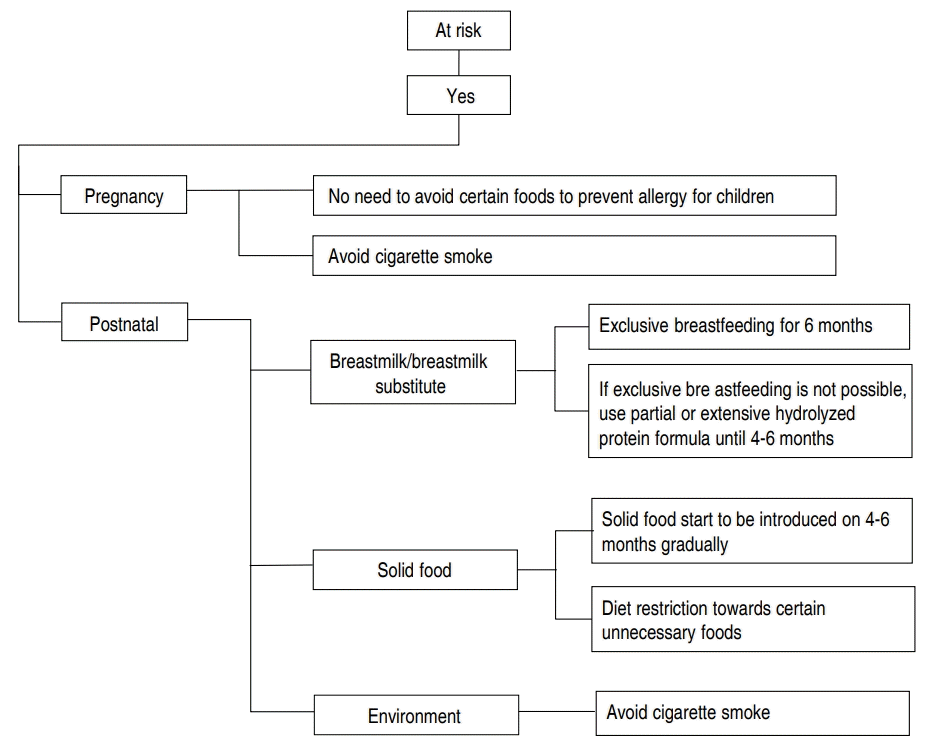
The Indonesian Pediatric Association guideline from 2015 recommends exclusive breastfeeding for 6 months as the best option to prevent primary allergy. In infants who are not breast-fed, hydrolyzed formulas, partial or extensive, are recommended until 4–6 months to help prevent AD in high-risk infants [18]. The algorithm of primary allergy prevention in Indonesia is shown in Fig. 1. The recommendation to use pHF for 4–6 months in nonbreastfed and at-risk babies is supported by recommendations from the US and Europe [19,20]. The recent Philippine guidelines recommend the use of pHF-W for at least 6 months to prevent the occurrence of allergies in high-risk non-breastfed infants [21].
An Indonesian health economic study by Botteman et al. [22] found that the use of 100% pHF-W in high-risk infants over the 6-year follow-up period would save cost up to IDR 35 million. Besides, the use of 100% pHF-W would lead to a 14% reduction in AD risk among high-risk infants. This result is in agreement with the analysis from five European countries (Denmark, France, Germany, Spain, and Switzerland), which found that the use of 100% pHF-W was cost-effective compared with standard CMF in the prevention of AD and cost saving compared with extensive hydrolyzed formula [23].
pHF for all nonbreastfed babies
The use of pHFs in at-risk infants was recommended in many guidelines. However, as previously mentioned, half of the infants who will develop allergies are not part of the at-risk group since the number of infants in the non-at-risk group is much larger [8]. Therefore, whether pHF should or could be considered as formula feeding for all nonbreastfed infants, independent of the genetic risk for allergy, is still under debate.
Based on current regulatory authorities, pHF is considered as a protein source that can be used in standard infant formula. The pHF also meets all nutrient requirements as required for standard CMF [5]. However, the European Food Safety Authority (EFSA)’s Scientific Opinion Panel on infant and follow-on formula states that the safety and suitability of each hydrolysate has to be demonstrated by clinical studies. One pHF-W has been approved for its safety and suitability by the Panel (EFSA, 2005f) and has been authorized for use by the Directive 2006/141/EC [24].
Long-term data on the growth and body composition of healthy infants taking pHF are limited. There was no difference in physical growth up to 6 months between presumed healthy infants fed with 100% pHF-W and those fed with standard CMF with intact protein [25]. The mean differences in the weight and length (z score) of 6-month male and female infants were -0.03 (95% CI, -0.15 to 0.10,) and -0.07 (95% CI, -0.18 to 0.04,) [9]. Other data from the GINI study showed that there was no significant difference in BMI (z score) of infants fed with 100% pHF-W in the first 4 months of life compared with those fed with standard CMF for up to 10 years [25]. Eight clinical trials were included in a recent review, seven of which used the same pHF-W [26]. Six out of 8 studies indicated a reduction in atopic manifestations using a specific pHF-W versus CMF in the first years of life [26]. The literature review confirmed that pHF-W supports normal growth in infants and suggests that the risk of AD may be reduced in not-fully breastfed infants when supplemented with a specific pHF-W during the first 4–6 months of life [26].
Data regarding the effects of pHF on the hormonal responses and serum metabolites of healthy term infants were limited. There is a theoretical concern that pHF may result in various absorption and metabolic responses compared with intact protein, because of the more rapid gastric emptying and thus more rapid release of higher plasma amino acid levels [27-29]. A rapid increase in plasma amino acids can be counteracted by a more rapid oxidation, which results in the maintenance of amino acids levels within safe limits. A higher postprandial aminoacidemia could result in higher secretion of insulin by β-cells [30,31]. In adults with type 2 diabetes, hydrolysates may in theory improve insulin secretion; however, this possibility still needs to be explored [31]. Infants born with a genetic risk of developing type 1 diabetes mellitus, and who were fed with pHF, did not develop an increased incidence of type 1 diabetes [32]. In addition, it could not be demonstrated that hydrolysates may protect infants from developing diabetes. Early exposure to complex dietary proteins may increase the risk of type 1 diabetes in children with genetic disease susceptibility. However, in infants at risk for type 1 diabetes, weaning to a hydrolyzed formula compared with a conventional formula did not reduce the cumulative incidence of type 1 diabetes after a median follow-up of 11.5 years [33].
Previous studies showed some beneficial effects of pHF-W on functional gastrointestinal manifestations. Infants fed with pHF with a high β-palmitic acid content, prebiotic oligosaccharides, and starch had lower incidence of regurgitation compared with the control group [34]. Moreover, infants in the pHF-W group had increased stool frequency after 1 week [34]. The positive effect of pHF on constipation was observed in another trial with a formula containing prebiotic and palmitic acid [36]. In yet another trial, pHF- W with prebiotic, palmitic acid, and low lactose significantly decreased infantile colic episodes after 1 week [35]. One prospective study reported a lower incidence of gastrointestinal manifestations (such as vomiting, diarrhea, constipation, or infantile colic) at the age of 6 months in infants fed with 100% pHF-W compared with those fed with standard formula [25]. Most clinical trials reporting the use of pHF-W in infants with functional gastrointestinal disorders included additional formula changes besides the change from intact to partially hydrolyzed protein, such as prebiotics, palmitic acid, and decreased lactose content [10,27].
All studies evaluating the effect of pHF showed some or no benefit, but never an increased risk of adverse effects. None of the data obtained suggest that pHF may be potentially harmful for healthy term infants. Based on the available data, an international expert consensus considered pHF as safe as CMF with intact protein [5,37]. pHF has been on the market for more than 25 years and is available in more than 90 countries; thus far, safety issues have not been reported in surveillance programs [10].
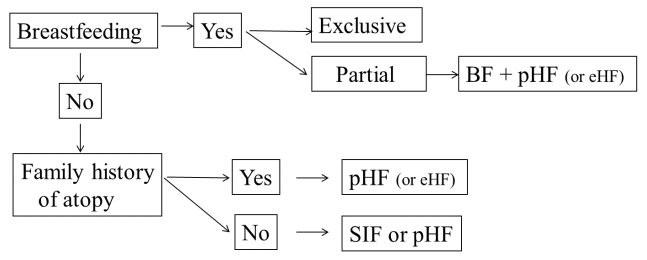
Based on the available data, pHF-W can be considered as an option in all nonbreastfed term healthy infants regardless of their allergy risk status (Fig. 2). From a cost/benefit perspective, the use of pHF is more cost-effective than the use of standard CMF, especially in preventing AD [21]. However, since all hydrolyzed formulas differ, the allergenicity, tolerability, effectiveness, and clinical impact of pHF differ. Therefore, each product should be tested. The impact of routine use of each pHF in larger populations on nonexclusively breastfed infants must be further evaluated to determine its benefits and cost-effectiveness ratio [37].
Conclusions
Breastfeeding is the best feeding for infants. In nonbreastfed infants, CMF provides adequate nutrition and supports normal growth and development. A pHF-W is a formula with proven efficacy in reducing AD and is recommended in some guidelines for high-risk infants to prevent the occurrence of atopic disease. However, since the number of infants who develop allergies is larger in the non-at-risk group than in the at-risk group, pHF should not be limited to the at-risk group. From the regulatory point of view, pHF- W meets all nutritional requirements and can be considered as an alternative to CMF with intact protein for all nonbreastfed infants. The use of this formula was also proven to be cost-effective in preventing AD compared with standard CMF. No data suggest that pHF is potentially harmful for healthy term infants. Therefore, the use of pHF-W for nonbreastfed and non-at-risk infants could be considered regardless of the allergy risk status.
Notes
YV has participated as a clinical investigator, advisory board member, consultant, and/or speaker for Abbott Nutrition, Astel Medica, Danone, Nestle Health Science, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, United Pharmaceuticals, and Wyeth. RWB and DK are employees of Nestle Nutrition Institute Indonesia. None of the other authors have conflict of interest.