Prognostic factors in children with extracranial germ cell tumors treated with cisplatin-based chemotherapy
Article information
Abstract
Purpose
To evaluate the outcomes and prognostic factors in children with extracranial germ cell tumors (GCTs) treated at a single institution.
Methods
Sixty-six children diagnosed with extracranial GCTs between 1996 and 2012 were included in the study. Primary treatment was surgical excision, followed by six cycles of cisplatin-based chemotherapy. The survival rates were compared according to the International Germ Cell Cancer Cooperative Group classification used for GCTs in adults to validate the classification guidelines for GCTs in children.
Results
The median patient age was 4.4 years. In 34 patients (51.5%), the primary tumor site was the gonad. Extragonadal GCTs were detected in 32 patients. The 5-year overall survival and event-free survival (EFS) were 92.0%±3.5% and 90.4%±3.7%, respectively. In univariate analysis, tumor histology, metastasis, and elevated alpha-fetoprotein were not prognostic factors in children with extracranial GCTs. However, EFS was poorer in patients with mediastinal disease (n=12, 66.7%±13.6 %) than in those with nonmediastinal disease (n=54, 96.0%±2.8%) (P=0.001). The 5-year EFS was lower in patients older than 10 years, (n=21, 80.0%±8.9%) compared with those younger than 10 years (n=45, 95.2%±3.3%) (P=0.04). Multivariate analysis identified the mediastinal tumor site as the only independent prognostic factor.
Conclusion
The prognosis of children with extracranial GCTs was favorable. However, nongerminomatous mediastinal tumors were associated with poor survival in children. Further research is needed to improve the prognosis of children with malignant mediastinal GCTs.
Introduction
Germ cell tumors (GCTs) are a heterogeneous group of tumors in terms of histology, site, and prognosis, despite originating from a single cell, namely a primordial germ cell. GCTs account for approximately 2%-4% of all tumors in children less than 15 years of age. Since the introduction of cisplatin-based chemotherapy, the survival of patients with GCT has improved by up to 80%12); however, the prognosis for some patients remains poor. In 20%-30% of adult GCT patients with high-risk features, the overall survival (OS) rate is less than 50%34). The International Germ Cell Cancer Cooperative Group (IGCCCG) classification is used worldwide to identify high-risk patients and to guide first-line treatment5). According to this classification, patients with nongerminomatous GCT who have mediastinal primary tumor, nonpulmonary visceral metastases, or high level of tumor markers are classified as high-risk patients. Clinical studies based on a dose-intense approach including high-dose chemotherapy with autologous stem cell rescue, reported a high response rate and favorable survival rates in high-risk patients678). However, the risk factors for GCT in adults might not have prognostic value in children910).
Limited data on the prognostic factors and outcomes of children with extracranial GCTs are currently available91112). However, the poor prognosis of a subgroup of patients underscores the need for risk-stratified intensive treatment strategies. In this context, we analyzed the data of children with extracranial GCTs treated at a single institution and compared the outcomes according to the IGCCCG classification used in adult GCTs to validate the classification guidelines in children.
Materials and methods
Between January 1996 and December 2012, 66 children and adolescents with extracranial GCTs were newly diagnosed in Samsung Medical Center. The first line of treatment was surgical excision of the primary tumor when feasible. This was followed by six cycles of chemotherapy consisting of cisplatin, etoposide and bleomycin (PEB). If the tumor resection was not feasible at diagnosis, resectability was re-evaluated after four cycles of PEB chemotherapy and patients received an additional two cycle of PEB chemotherapy after tumor resection. Patients with disseminated disease or unresectable tumors were further treated with six cycles of maintenance chemotherapy consisting of vinblastine and doxorubicin when residual disease was present after the completion of six cycles of PEB chemotherapy. Patients with mature teratoma were treated with tumor resection without adjuvant chemotherapy, whereas patients with immature teratoma were treated with chemotherapy when tumors showed malignant features or histologic grade 2 with elevated tumor marker or grade 3.
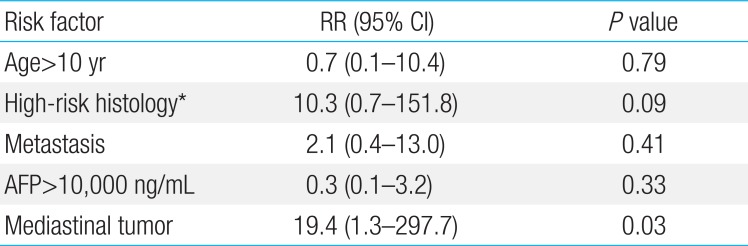
In all patients, the initial diagnostic procedures included computed tomography or magnetic resonance imaging and measurement of tumor markers in serum. If the levels of tumor markers (alpha-fetoprotein [AFP], human chorionic gonadotropin [HCG]) were elevated at diagnosis, they were measured before each cycle of chemotherapy thereafter. To validate the high risk factors based on the IGCCCG classification in children with extracranial GCTs, survival was compared according to the risk factors such as primary mediastinal nongerminomatous GCTs, nonpulmonary visceral metastasis, AFP>10,000 ng/mL, HCG>50,000 IU/L, and lactate dehydrogenase (LD)>10×normal.
Response rates were compared using the Pearson chi-square test or Fisher exact test. The Kaplan-Meier method was used to estimate OS and event-free survival (EFS). Multivariate analysis for identifying independent prognostic factors was made using Cox proportional hazards regression model for the analysis of times to events.
Results
1. Patient characteristics
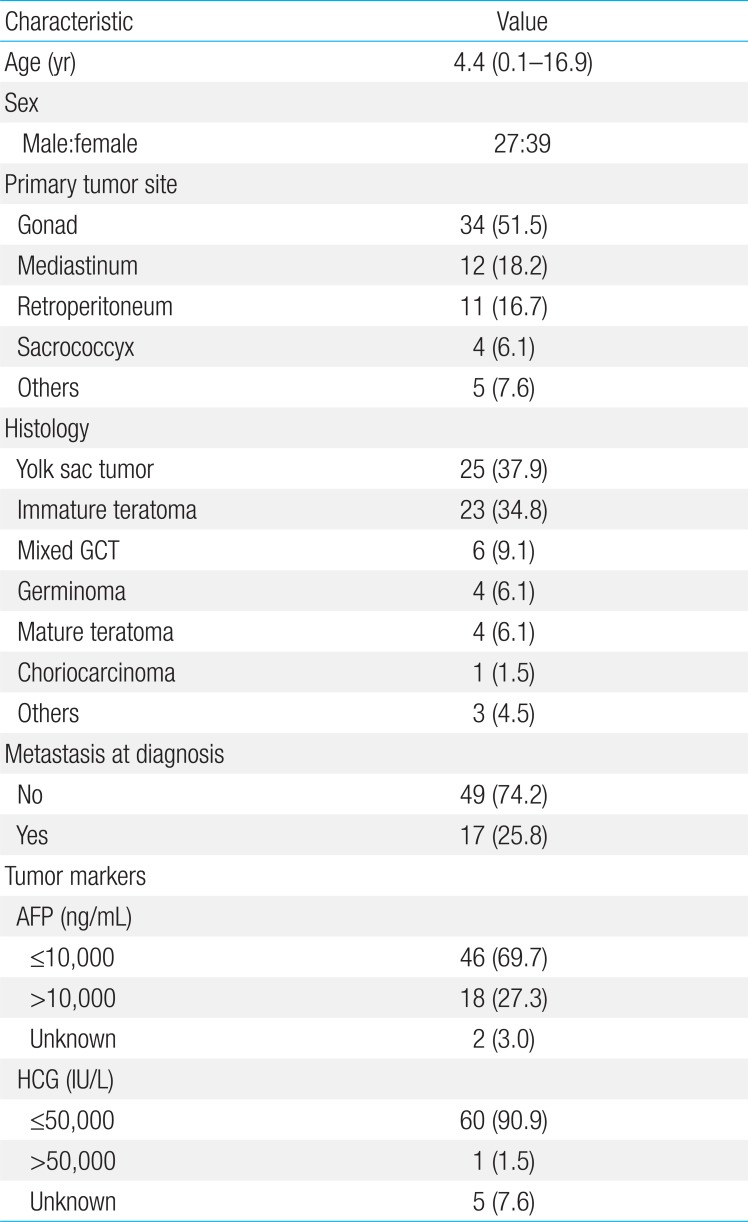
The median age of extracranial GCT patients at diagnosis was 4.4 years (range, 0.1-16.9 years) (Table 1). The primary tumor site was the gonad in 34 patients (51.5%), and extragonadal GCTs were detected in 32 patients (48.5%). The predominant histology was yolk sac tumor (n=25, 37.9%). When we examined tumor makers, we found that one patient showed a HCG level higher than that classified as high-risk (50,000>IU/L) by the IGCCCG.
2. Treatment
Forty-four patients underwent tumor resection at the time of diagnosis and 15 patients underwent delayed surgery after chemotherapy. The remaining seven patients, in whom the primary tumors were located in extragonadal sites, including sacrococcygeal region (n=2), presacral region (n=1), neck (n=1), prostate (n=1), orbit (n=1), and vagina (n=1), did not undergo surgery. Four out of seven patients had metastatic disease at diagnosis. Chemotherapy was not administered in four patients with mature teratoma, three patients with stage I testicular yolk sac tumor, and three patients with low grade immature teratoma.
3. Outcomes
The 5-year OS and EFS rates were 92.0%±3.5% and 90.4%±3.7%, respectively (Fig. 1). Relapse occurred in five patients, of whom one underwent high-dose chemotherapy and autologous stem cell rescue and is alive. The remaining four patients died from progressive disease. Univariate analysis according to the risk factors revealed no differences in the 5-year EFS between the patients with mature teratoma, immature teratoma, or germinoma (96.6%±3.4 %) and those with other histologies (84.7%±6.3%, P=0.11). AFP levels at the time of diagnosis were not correlated with prognosis (Table 2). Metastatic disease at the time of diagnosis was not a significant prognostic factor, although the 5-year EFS was 93.5%±3.6% in patients without metastases and 81.9%±9.5% in patients with metastases (P=0.16). In addition, the EFS of children with nonpulmonary visceral metastases was 90.9%±8.7%, whereas that of children with pulmonary metastases was 62.5%±21.3% (P=0.21) (Table 2). However, EFS was poorer in patients with mediastinal disease than in those with nonmediastinal disease (Table 2). The 5-year EFS was worse in patients older than 10 years of age (n=21, 80.0%±8.9%) than in those younger than 10 years of age (n=45, 95.2%±3.3%) (P=0.04). Multivariate analysis identified a mediastinal tumor site as the only independent prognostic factor (Table 3).
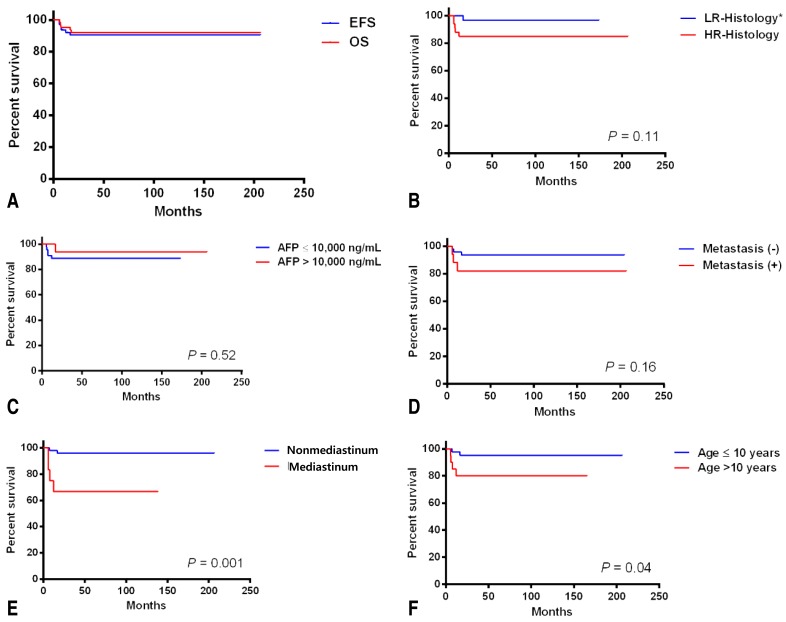
(A) The 5-year event-free survival (EFS) and overall survival (OS). (B-F) The 5-year EFS according to the risk factors. *LR-histology includes mature teratoma, immature teratoma, and germinoma. HR-histology includes yolk sac tumor, choriocarcinoma, and other mixed germ cell tumors. LR, low-risk; HR, high-risk; AFP, alpha-fetoprotein.
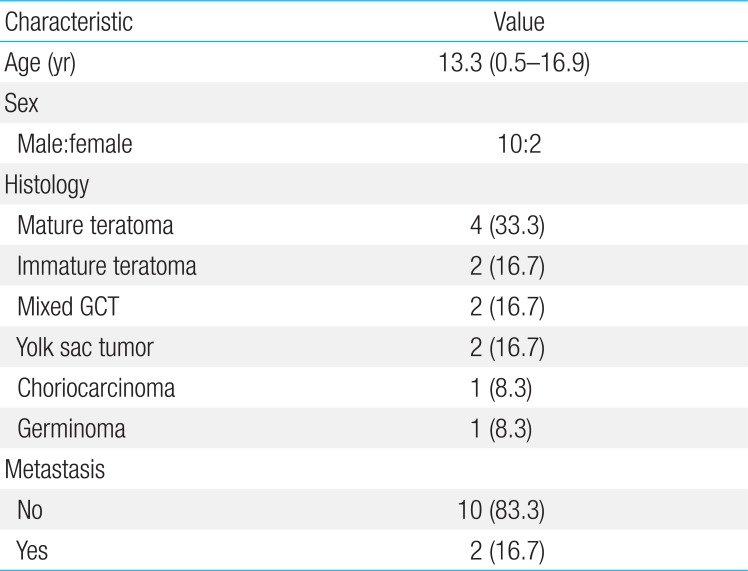
4. Mediastinal tumors
The median age of the patients with mediastinal GCTs were higher than that of patients with nonmediastinal GCTs (13.3 years vs. 3.8 years, P=0.02) (Table 4). The ratio of males-to-females was higher in patients with mediastinal GCTs (M:F=10:2) than in those with nonmediastinal GCTs (M:F=17:37; P=0.002). The 5-year EFS of patients with germinoma and mature teratoma (n=5) was 100%, whereas that of patients with nongerminomatous GCTs (n=7) was 42.9%±18.7% (P=0.05).
Discussion
The prognosis of children with extracranial GCTs has improved significantly since the introduction of cisplatin-based chemotherapy. However, the prognostic factors vary among studies1314). Despite the use of risk-based strategies, there is no consensus regarding risk stratification guidelines in children with extracranial GCTs. On the other hand, the IGCCCG classification has been used in adults to stratify high-risk patients with OS rates of less than 50%. In the present study, we evaluated the outcomes of children with extracranial GCTs treated with cisplatin-based chemotherapy and validated the IGCCCG classification guideline in pediatric extracranial GCTs.
The results showed that nonpulmonary visceral metastases and high levels of tumor markers were not associated with a poor prognosis. However, the outcomes of children with mediastinal nongerminomatous GCTs were poor, with EFS rates less than 50%. Previous studies on children with GCTs reported that primary tumor site, tumor stage, histology, and the levels of tumor markers were significant prognostic factors, especially in the precisplatin era. However, those factors lost their prognostic relevance after the introduction of cisplatin. In adults, an AFP level higher than 10,000 ng/mL is an independent poor prognostic factor151617). However, in pediatric studies, the prognostic significance of AFP levels varies91118). This factor may not be relevant in children with GCTs primarily treated with intensive cisplatin-based chemotherapy911). The risk factors in adult GCTs might not have prognostic value in pediatric GCTs. This variability could be explained by age-related cytogenetic differences. GCTs in adults are characterized by an i(12p) or a 12p amplification in >90%, whereas in children, different regions of gain (+1q, +3, +20q, and +x) and loss (-4q, -6q, and -y) have been reported19202122).
Pediatric extracranial GCTs are characterized by a high frequency of nongonadal tumors, which comprise approximately 50% of all GCTs. Mediastinal tumors represent an important subgroup that accounts for 3.5% of all pediatric GCTs. A study on mediastinal GCTs in adults showed that 48 of 88 patients (55%) with malignant nonseminomatous GCTs achieved a disease-free status and 31 of 88 patients (35%) were long-term survivors. Therefore, the mediastinal site is considered an adverse prognostic factor for malignant nonseminomatous GCTs in adults. A study conducted by the French Society of Pediatric Oncology on localized malignant nonseminomatous GCTs in children reported that the 3-year failure-free survival was 57% and an analysis of prognostic factors suggested that a mediastinal site was an unfavorable prognostic predictor11). Among the 93 children included in the Children's Cancer Study Group protocol, 17 had malignant mediastinal tumors. Events occurred in 11 of 17 patients, and the mediastinal site was associated with an inferior prognosis compared with the sacrococcygeal and other nongonadal sites23). However, a recent study conducted in Germany suggests that the prognosis in children with primary mediastinal malignant GCTs improves with delayed resection after preoperative chemotherapy10). In the present study, patients with primary mediastinal GCTs had a worse prognosis than those with GCTs in other sites, and the mediastinal site was the only independent prognostic factor (Table 3). In addition, clinical differences were observed between GCTs arising in the mediastinum and those originating in other sites.
In conclusion, the prognosis of children with GCTs was favorable. The risk factor for adults GCTs might not have prognostic value in children with GCTs. However, pediatric nongerminomatous mediastinal tumors were associated with poor survival, which is similar to the pattern in adults. Further research is needed to improve the prognosis of the children with malignant mediastinal GCTs. A strategy comprising front-line high-dose chemotherapy or a novel chemotherapy regimen with paclitaxel should be evaluated in future studies172425).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.