Necrotizing fasciitis and streptococcal toxic shock syndrome secondary to varicella in a healthy child
Article information
Abstract
Varicella is usually considered to be a benign disease in healthy children; however, serious complications can occur such as necrotizing fasciitis and toxic shock syndrome. We describe a 38-month-old girl with necrotizing fasciitis and streptococcal toxic shock syndrome following varicella. She was previously healthy and vaccinated against varicella at 12 months of age. She had been diagnosed with varicella three days prior to presenting at our facility; she developed fever, vomiting, and painful swelling on her left flank. Her skin lesions worsened, she became lethargic, and had episodes of hypotension and coagulopathy. Necrotizing fasciitis on the left abdominal wall, buttocks, and left thigh was diagnosed by magnetic resonance imaging, and group A Streptococcus was isolated from a tissue culture. She was diagnosed as necrotizing fasciitis and streptococcal toxic shock syndrome, and successfully treated with repeated surgical debridement and fasciotomy, in addition to intensive antibiotics. Our experience suggests that necrotizing fasciitis in patients with varicella should be considered to be a rare complication even with widespread vaccine use. Early diagnosis and intensive treatment are required to prevent a fatal outcome.
Introduction
Varicella is a systemic infectious disease caused by the varicella-zoster virus (VZV). The clinical manifestations are characterized by a generalized, pruritic, vesicular rash in various stages of development, mild fever, and malaise. It is usually considered to be a benign disease in healthy children. However, life-threatening infections and severe complications can occur such as bacterial superinfection of the skin lesions and toxic shock syndrome. Herein, we report a case of necrotizing fasciitis (NF) as a complication of varicella in a vaccinated child; this occurred despite the fact that the number of varicella cases and associated deaths have decreased dramatically in many nations since the introduction of the varicella vaccine1,2).
Case report
A previously healthy 38-month-old girl visited the Emergency Department with fever, irritability, and a chief complaint of increasing pain and swelling in the left flank. She had been diagnosed with varicella at a local clinic three days ago, despite having been vaccinated against varicella. She had been vomiting for one day and developed fever, abdominal pain in the left lower quadrant, and painful swelling of the left flank. At the time of admission, body temperature was 38.9℃, pulse rate was 128 beats per minute, respiratory rate was 24 breaths per minute, and blood pressure was 92/50 mmHg. Ruptured blisters, a macular rash, including some lesions with painful purplish swelling, were noticed on the skin of her left flank extending to the buttocks (Fig. 1). Laboratory investigation showed a hemoglobin concentration of 11.7 g/dL, white blood cell count 5,250/µL (84% neutrophils, 8% lymphocytes, 1% monocytes), platelet count of 80,000/µL, and C-reactive protein of 17.21 mg/dL. VZV IgM and IgG were all positive. An abdominal computed tomography (CT) scan showed extensive subcutaneous soft tissue swelling of the left anterior abdominal wall, flank, and inguinal area (Fig. 2A). The patient was treated with hydration and intravenous antibiotics (ceftriaxone). However, the purplish swelling with macular rash lesion rapidly extended to the back and left thigh over the following two days; in addition, it exhibited necrotic change. She became lethargic and oliguric (0.7 mL/kg/hr). Her blood pressure decreased to 84/27 mmHg. Aggressive fluid therapy and transfusion were administered because she had episodes of hypotension. Laboratory investigation revealed disseminated intravascular coagulation (hemoglobin concentration, 8.6 g/dL; white blood cell count, 10,340/µL [62.1% neutrophils, 28.8% lymphocytes, 6.2% monocytes]; platelet count, 53,000/µL; prothrombin time, 15.3 seconds; partial thromboplastin time, 45.8 seconds; fibrinogen, 446 mg/dL; D-dimer, 3.94 µg/mL; and fibrin degradation products, 13.64 µg/mL). Follow-up abdominal CT scan showed an aggravated, extensive subcutaneous soft tissue swelling in the left anterior abdominal wall, both flanks, back, and inguinal area (Fig. 2B). Magnetic resonance imaging (MRI) was performed and it showed NF on left abdominal wall, buttocks, and left thigh (Fig. 3). In addition to intensive antibiotics (vancomycin and clindamycin) treatment, necrotic skin and soft tissue were removed after repeated surgical debridement, local dressing, and fasciotomy for three times. Group A Streptococcus (GAS) was isolated from the tissue culture, which was susceptible to penicillin and clindamycin, but no bacteria was isolated from the blood cultures. Antibiotics were changed to ampicillin/sulbactam and clindamycin. Isolation of GAS from tissue culture and the clinical signs such as hypotension, coagulopathy and soft tissue necrosis led to the diagnosis of streptococcal toxic shock syndrome. Antibiotics treatment and supportive care were continued. After five weeks the patient was discharged in good health and her skin lesions were healing.
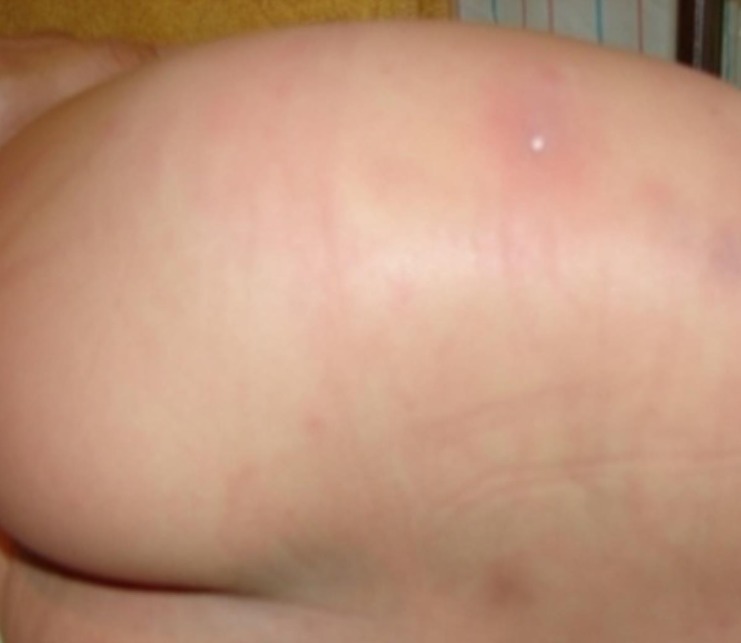
Skin lesion of the patient at the time of admission. Erythematous rash and purplish swelling with ruptured blisters on left flank extending to the buttocks.
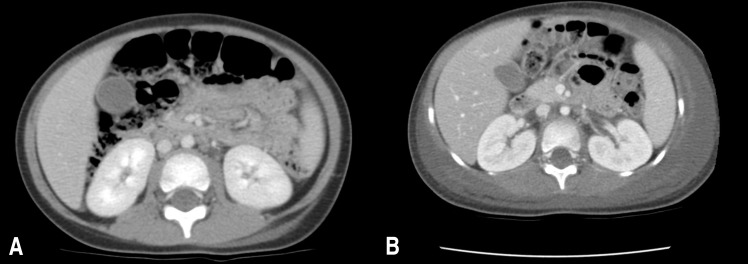
Abdominal computed tomography (CT) scan of the patient. (A) At the time of admission, it showed extensive subcutaneous soft tissue swelling of the left anterior abdominal wall, flank, and inguinal area. (B) At the 3rd day of admission, follow-up abdominal CT scan showed an aggravated, extensive subcutaneous soft tissue swelling in the left anterior abdominal wall, both flanks, back, and inguinal area.
Discussion
Varicella is usually considered a self-limited disease, and severe complications were previously thought to be related to immunodeficiency or underlying disease. However, healthy children can also experience severe complications such as neurological disorders, bacterial superinfections, or hematologic complications3). In France, bacterial superinfection was reported in 50.3%, NF in 0.8%, and shock in 0.7% of the cases4). In Germany, infectious complications of varicella were reported only 38.6% of the cases in children up to the age of 16. However, they were present in the majority of younger children under 5 years of age. Invasive disease attributable to GAS infection after varicella was identified as a major risk factor for an unfavorable outcome3). Invasive GAS infection following varicella in children occurred in 6%-30% of the cases in North America in the 1990s1,5). In Canada, children with invasive GAS infection who recently had varicella were more likely to have NF. The percentage of varicella associated with invasive GAS hospitalizations decreased from 27% in the prevaccination era, to 16% during vaccine implementation, and 2% after widespread vaccine use1).
A live attenuated varicella vaccine was developed in Japan in 1974. Since the introduction of varicella vaccine, the number of varicella cases and serious complications has decreased dramatically2). Since 2005 in South Korea, varicella vaccination of children 12-15 months of age has been added to the national immunization program; the immunization rate was estimated to be greater than 80% in 20076). Despite this high vaccine coverage rate, outbreaks of varicella or herpes zoster have still occurred because of breakthrough infections or waning immunity7). Furthermore, fatal cases of streptococcal toxic shock syndrome or invasive GAS infections still occur in some nations despite the immunization strategies1). In this case, our patient had received a single dose of the live attenuated varicella vaccine at 12 months of age. The vaccine was made from the MAV/06 strain, which originated from a Korean child with varicella7). We could not distinguish between the VZV wild-type strain and the vaccine-type strain in this patient. Most cases of varicella or herpes zoster caused by VZV vaccine occurred in immunocompromised children, because underlying immunodeficiency might trigger reactivation of the vaccine strain8,9). Although cases of herpes zoster after vaccination in immunocompetent children were recently reported, the symptoms were not severe and no complications were reported9,10). In this case, waning immunity might be considered to trigger development of the serious complication of varicella infection in the patient with breakthrough infection.
In conclusion, although the risk of invasive GAS disease including NF in children with varicella is decreasing, severe complications can occur even in vaccinated children. Therefore, NF should be suspected in any child with a recent history of varicella and a complaint of increasing pain and swelling associated with fever or lethargy. Early diagnosis and intensive treatment are required to prevent a fatal outcome.
Notes
No potential conflict of interest relevant to this article was reported.
