Vitamin D status and childhood health
Article information
Abstract
Vitamin D is an essential component of bone and mineral metabolism; its deficiency causes growth retardation and skeletal deformities in children and osteomalacia and osteoporosis in adults. Hypovitaminosis D (vitamin D insufficiency or deficiency) is observed not only in adults but also in infants, children, and adolescents. Previous studies suggest that sufficient serum vitamin D levels should be maintained in order to enhance normal calcification of the growth plate and bone mineralization. Moreover, emerging evidence supports an association between 25-hydroxyvitamin D (25[OH]D) levels and immune function, respiratory diseases, obesity, metabolic syndrome, insulin resistance, infection, allergy, cancers, and cardiovascular diseases in pediatric and adolescent populations. The risk factors for vitamin D insufficiency or deficiency in the pediatric population are season (winter), insufficient time spent outdoors, ethnicity (non-white), older age, more advanced stage of puberty, obesity, low milk consumption, low socioeconomic status, and female gender. It is recommended that all infants, children, and adolescents have a minimum daily intake of 400 IU (10 µg) of vitamin D. Since the vitamin D status of the newborn is highly related to maternal vitamin D levels, optimal vitamin D levels in the mother during pregnancy should be maintained. In conclusion, given the important role of vitamin D in childhood health, more time spent in outdoor activity (for sunlight exposure) and vitamin D supplementation may be necessary for optimal health in infants, children, and adolescents.
Introduction
Vitamin D, the sunshine vitamin, has long been recognized as essential for bone and mineral metabolism. Deficiency of vitamin D causes osteomalacia, leading to growth retardation and skeletal deformities in children and osteoporosis in adults1). Ultraviolet B wavelengths in sunlight trigger vitamin D synthesis in the skin by converting 7-dehydrocholesterol to cholecalciferol (vitamin D3), which enters the circulation1).
Sun exposure is the main source of vitamin D in humans, as only a small amount is obtained from the diet1). The major dietary sources of vitamin D are oily fish, dairy products, eggs, and meat. Vitamin D3 is modified by 2 hydroxylation steps to the active form. In the liver, vitamin D3 is converted to 25-hydroxyvitamin D (25[OH]D), the major circulating form and best indicator of vitamin D status. In the kidney, 25(OH)D is converted to 1,25-dihydroxyvitamin D (1,25[OH]2D), the biologically active metabolite. Further, 1,25(OH)2D acts by binding to the nuclear vitamin D receptor (VDR) within cells (Fig. 1). The VDR is broadly expressed throughout the body. The VDR is found in the endocrine glands (pituitary, pancreas, parathyroid, gonads, and placenta)2) and in cardiovascular tissues such as endothelial cells, vascular smooth muscle cells, and cardiomyocytes3). The VDR has also been found in hematolymphopoietic cells, and vitamin D has been shown to regulate cell differentiation and the production of interleukins and cytokines1,2). In light of the near universal expression of the VDR across tissues, vitamin D would be expected to play a role in diseases other than those of bone. The wide variety of nonskeletal diseases in which vitamin D is important include cardiovascular diseases3), obesity4), metabolic syndrome5), insulin resistance6), infection7), allergy8,9), some forms of cancers10-12), and autoimmune diseases13).
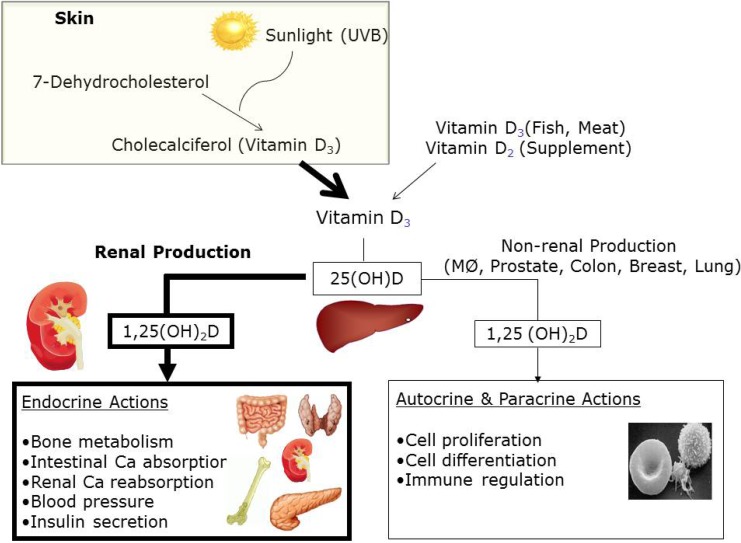
Schematic of vitamin D metabolism and actions in humans. UV-B, ultraviolet B; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; MØ, macrophage.
Although vitamin D deficiency is known to be prevalent in adults, epidemiologic data on the prevalence of vitamin D deficiency are lacking in Korean infants, children, and adolescents. Recent studies suggest that vitamin D insufficiency or deficiency may have a significant impact on childhood health14,15). There are several epidemiologic studies suggesting that vitamin D plays an important role in the prevention and management of various pediatric diseases. Thus, given the important role of vitamin D in childhood health, this review will focus on the impact of vitamin D on health and disease in childhood and adolescence.
Epidemiology of vitamin D status
1. Diagnostic criteria of vitamin D insufficiency or deficiency
No consensus on the definition of vitamin D deficiency exists; however, most clinicians and researchers agree on the following stratifications based on the serum concentration of 25(OH)D: deficiency, <50.0 nmol/L or <20.0 ng/mL; insufficiency, 50.0-74.9 nmol/L or 20.0-29.9 ng/mL; and sufficiency, ≥75.0 nmol/L or ≥30.0 ng/mL1,6,16,17).
2. Prevalence of vitamin D insufficiency or deficiency
Epidemiologic data on the prevalence of vitamin D insufficiency or deficiency in children are sparse or incomplete in most countries. However, it is an important public health problem in both developed and developing countries, with an estimated prevalence of 29-100% in children and adolescents4,6,18-22). A study based on the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV), conducted in 2008, determined that among 3,047 male subjects and 3,878 female subjects aged ≥10 years, 86.8% of male subjects and 93.3% of female subjects had a serum 25(OH)D level of less than 30 ng/mL. This indicated that vitamin D insufficiency or deficiency was highly prevalent in Koreans across all age groups23). In line with these data, a study focusing on adolescents aged 12-13 years reported that 98.9% of boys and 100% of girls showed vitamin D insufficiency or deficiency; 5.3% had 25(OH)D insufficiency (20.0-29.9 ng/mL), 94.2% had deficient concentrations (<20.0 ng/mL), and only 1 boy (1.1%) had optimal concentrations of 25(OH)D6). A recent study in Hangzhou, China investigated the vitamin D status of 6,008 Chinese children aged 1 month to 16 years18). They classified the subjects into subgroups according to their age: 0-1 year, 2-5 years, 6-11 years, and 12-16 years representing infancy, preschool, school age, and adolescence stages, respectively. They found that in winter and spring, more than 50% of school age children and adolescents had a vitamin D deficiency, and in winter, 100% of the adolescents and 93.7% of school age children had vitamin D insufficiency18).
3. Predictors of vitamin D insufficiency or deficiency
The main risk factors for vitamin D insufficiency or deficiency in the pediatric population are season (winter), insufficient time spent outdoors, non-white ethnicity, older age, more advanced stage of puberty, obesity, low milk consumption, low socioeconomic status, and female gender19). Seasonal variability has an important impact on vitamin D status24); the vitamin D levels are lowest in winter, compared with spring, summer, and autumn20) because most vitamin D is produced following exposure to sunlight. A recent study based on the KNHANES IV 2008-2009 examining a total of 1,510 healthy adolescents aged 12-18 years (806 male subjects and 704 female subjects; mean age, 14.7 years) assessed that the prevalence of vitamin D deficiency (25[OH]D<20.0 ng/mL) was 89.1% in spring, 53.7% in summer, 63.9% in autumn, and 90.5% in winter, and that independent predictors for low vitamin D status were season (winter), higher education level, and a lack of vitamin D supplementation22). Young people tend to spend more time indoors and less time outdoors as compared with older people23,25). For example, most Korean adolescents spend most of the daylight hours studying at school and private cram schools in order to do well in the entrance examinations for prestigious high schools and universities26). Therefore, they may not have enough exposure to sunlight for optimal cutaneous production of vitamin D.
A study conducted in the United States speculated that American adolescents tend to consume less dairy products27). Girls are at particular risk for low vitamin D status, which has been ascribed to lifestyle factors such as frequent use of sunscreen or coverage of skin by clothing that could affect cutaneous synthesis of vitamin D6). Mounting evidence also suggests that there are ethnic differences in vitamin D levels20,21). Black subjects have lower plasma 25(OH)D concentrations than do white subjects 20,21,28-31). Low vitamin D status in black individuals may be attributed mainly to decreased skin synthesis of vitamin D due to greater skin pigmentation32) or to the high prevalence of obesity in black populations25,33,34). However, there are no epidemiologic data comparing Asians with whites or blacks with regard to serum vitamin D levels.
Vitamin D insufficiency/deficiency and childhood health
1. Vitamin D insufficiency/deficiency and childhood bone health
The critical role of vitamin D in skeletal mineralization was the first of its physiological functions to be identified. Vitamin D is required for normal calcification of the growth plate and bone mineralization35). Vitamin D deficiency results in osteomalacia, which leads to growth retardation and the development of skeletal deformities such as rickets in children. The major role of vitamin D in maintaining bone health is to ensure normal calcium and phosphate levels in the blood. Vitamin D, through its interaction with the VDR, increases the efficiency of intestinal calcium absorption to 30-40% and phosphorus absorption to approximately 80%1). The calcium demands of the individual affect intestinal absorption of dietary calcium. Children have higher calcium demands than adults; they require a positive calcium balance to assure adequate calcium for the mineralization of growing bone. One study reported that if the vitamin D level was 30 ng/mL or less, there was a significant decrease in intestinal calcium absorption36), which correlated to increased circulating parathyroid hormone (PTH)37). Without an adequate amount of calcium-phosphorus product (the value for calcium times the value for serum phosphorus), mineralization of the collagen matrix is diminished, resulting in the development of rickets in children38) and osteomalacia in adults39). A recent study assessed serum 25(OH)D and its relationship to PTH in young children in New Zealand40). They found that in order to maintain low levels of PTH, 25(OH)D concentrations should be more than 60-65 nmol/L40). Thus, it is important to maintain sufficient serum vitamin D levels in order to enhance normal calcification of the growth plate and bone mineralization.
2. Vitamin D insufficiency/deficiency and respiratory diseases
Emerging evidence suggests that vitamin D plays an essential role in the modulation of the immune responses. In addition, vitamin D has been recognized as an anti-infective agent due to its effects on innate and adaptive immune responses. Thus, the question has been raised as to whether vitamin D status influences the risk of respiratory tract infections such as common viral infections or pulmonary tuberculosis in children. Recently, a randomized placebo-controlled trial conducted in Japan suggested that vitamin D3 supplementation during the winter season may be effective in reducing the incidence of seasonal influenza in school children41). In contrast, a study by Jorde et al.42) found that supplementation with vitamin D3 did not have beneficial effects on the incidence and severity of influenza-like disease as diagnosed by questionnaires.
Several studies conducted in different ethnic groups have found a close association between vitamin D deficiency and an increased risk of tuberculosis43-45). In addition, a recent meta-analysis found that low serum levels of vitamin D were associated with a high risk of active tuberculosis46). In contrast, a Cochrane review reported that vitamin D supplementation in patients diagnosed with tuberculosis did not alter the rates of sputum smear conversion in adults or body weight in children47).
Vitamin D insufficiency or deficiency is highly prevalent among children with bronchial asthma. Lewis et al.48) reported that the serum concentration of 25(OH)D was positively associated with asthma control in children. Another recent study showed that lower vitamin D levels in 86 children with severe, therapy-resistant asthma in the United Kingdom were associated with worsened asthma control and lung function49). A randomized placebo-controlled prospective study by Majak et al.50) showed that vitamin D supplementation (500 IU of cholecalciferol) daily for 6 months reduced the risk of bronchial asthma exacerbation triggered by acute respiratory tract infection. These observational and clinical studies indicate a beneficial effect of vitamin D supplementation in vitamin D deficient children with asthma or acute or chronic respiratory infections.
3. Vitamin D insufficiency/deficiency and obesity-related risk factors
The prevalence of childhood and adolescent obesity is increasing worldwide and in Korea, and this increased prevalence of obesity among children and adolescents may lead to increased risk of diabetes, hypertension, and cardiovascular disease51). These risk factors begin in childhood and track into adulthood. Some obesity-related health consequences have been correlated with vitamin D deficiency or insufficiency5,52-54), and recent reports have suggested that vitamin D supplementation may reduce these risks, although the data are inconsistent55,56). The inverse association between higher body fat and low vitamin D concentrations may be ascribed to sequestration of the fat-soluble vitamin D within adipose tissues57). It may also be explained by the fact that increased leptin levels induced by excess body fat may inhibit renal synthesis of the active form of vitamin D58). Other evidence suggests that low dietary vitamin D and decreased sun exposure due to fewer outdoor activities are risk factors for the low vitamin D levels observed in obese subjects4,20).
Studies have suggested that vitamin D may directly regulate insulin secretion by binding to pancreatic β-cell VDRs59), and may indirectly affect pancreatic β-cell function by regulating extracellular calcium concentrations59); these data suggest that low vitamin D levels may be associated with insulin resistance. In addition, a recent epidemiologic study assessing a population of French-Canadian children and adolescents (878 boys and 867 girls) found a positive correlation between 25(OH)D levels and blood levels of total cholesterol, apolipoprotein A1, apolipoprotein B, and triglycerides54). In line with these results, we recently reported that serum 25(OH)D levels were inversely associated with the homeostatic model assessment of insulin resistance, triglycerides, and low-density lipoprotein cholesterol in Korean adolescents aged 12-13 years6). Moreover, a randomized clinical trial determining the efficacy of vitamin D supplementation in obese adolescents showed that supplementation of 4,000 IU vitamin D3/day for 6 months significantly improved insulin sensitivity60). Taken together, these findings suggest that public health interventions such as outdoor activity and vitamin D supplementation, which enhance vitamin D status, may improve the health of adolescents with metabolic problems.
4. Effects of maternal vitamin D insufficiency/deficiency on infants and children
Studies have suggested that vitamin D concentrations in the fetus and newborn are highly dependent on and significantly related to maternal serum 25(OH)D concentrations61-64). Therefore, adequate vitamin D levels in the mother during pregnancy promote better bone health in the offspring65). In a prospective study of 198 children in the United Kingdom, decreased 25(OH)D concentrations in mothers during late pregnancy were correlated with decreased whole-body and lumbar spine bone mineral content in children at the age of 9 years65). This finding may be explained by previous studies suggesting that higher vitamin D levels or appropriate vitamin D intake in pregnant women is associated with increased infant birth weight66,67). In contrast, other observational studies showed no correlation between maternal vitamin D status and infant birth weight68,69).
There are prospective studies suggesting that higher maternal vitamin D status during pregnancy may lower the development of wheezing episodes in infancy70,71) or lower respiratory tract infections72). One study found that higher maternal vitamin D status during pregnancy increased the development of atopic dermatitis or asthma in the offspring69), and another found no significant associations between maternal late-pregnancy vitamin D status and either asthma or wheezing at the age of 6 years73). In general, the observational evidence linking low maternal vitamin D status during pregnancy to an increase in the risk of allergic diseases in the offspring seems more convincing.
Recommendation of vitamin D supplementation for infants, children, and adolescents
Recently, the American Academy of Pediatrics (AAP) recommended that all infants (beginning soon after birth), children, and adolescents have a minimum daily intake of 400 IU (10 µg) of vitamin D74), which is two times the previous recommended dose of vitamin D supplementation suggested in a 2003 clinical report from the AAP. The daily vitamin D recommendation for women during pregnancy and lactation is 600 IU (15 µg) according to the U.S. Food and Drug Administration. In 2011, the U.S. Endocrine Task Force on Vitamin D reported that 600 IU/day may not be sufficient to correct vitamin D deficiency in pregnant and lactating women, and therefore suggested 1,500-2,000 IU/day for pregnant and lactating women with vitamin D deficiency75). It is not known, however, how much amount of vitamin D supplementation is needed for Korean children and pregnant women in order to maintain adequate concentrations of vitamin D. Considering the high prevalence of vitamin D insufficiency or deficiency in Koreans, especially children and adolscents6,22-24), this issue needs to be addressed urgently by future studies.
Conclusions
Vitamin D insufficiency or deficiency is a very common health problem in children and adolescents. Emerging evidence suggests a close association between 25(OH)D levels and immune function, insulin resistance, and cardiometabolic diseases, which extends the consequences of vitamin D deficiency beyond those involving mineral and bone metabolism. From a perspective of integrative childhood care, it may be advantageous to recommend more time spent in outdoor activity for sunlight exposure and increased vitamin D supplementation to achieve optimal health in children and adolescents.
Notes
No potential conflict of interest relevant to this article was reported.