Evaluation of immunogenicity of the 2008-2009 seasonal influenza vaccines by microneutralization test
Article information
Abstract
Purpose
For evaluating the immunogenicity of an influenza vaccine, the microneutralization (MN) test has a higher sensitivity and specificity as compared to the hemagglutination inhibition (HI) test. However, the MN test is more time consuming and is difficult to standardize. We performed the MN test to determine its usefulness as an alternative or complementary test to the HI test for evaluating the immunogenicity of influenza vaccines.
Methods
We compared the MN test with the HI test using 50 paired samples taken from a previous clinical study (2008-2009) in Korean children under 18 years of age.
Results
The linear correlation coefficients of the 2 tests for H3N2, H1N1, and influenza B were 0.69, 0.70, and 0.66, respectively. We identified a high index of coincidence between the 2 tests. For an influenza vaccine, the postvaccination seroprotection rates and seroconversion rates determined by the MN test were 78.0% and 96.0%, 90% and 42.0%, and 42.0% and 48.0% for H3N2, H1N1, and influenza B, respectively. Geometric mean titer fold increases of H3N2, H1N1, and influenza B were 2.89, 5.04, and 4.29, respectively, and were 2.5-fold higher. We obtained good results in the evaluation of the immunogenicity of the 2008-2009 seasonal influenza vaccines.
Conclusion
We found that the MN test was as effective as the HI test. Therefore, we suggest that the MN test can be used as an alternative or complementary test to the HI test for evaluating the immunogenicity of influenza vaccines.
Introduction
Influenza is caused by the influenza virus and is one of the most serious and important infectious diseases in children and adults1-4). Vaccination is the best influenza prevention method, even though annual vaccination is necessary because influenza virus strains change frequently and protective immunity lasts only about half a year. Some insist on the necessity for annual evaluation of the immunogenicity of influenza vaccines. For example, immunogenicity of influenza vaccine has been evaluated annually in Europe5). If reasonable correlation of immunogenicity of vaccine with prevention of a specific disease is established in a number of studies, we can begin evaluating immunogenicity of a vaccine instead of evaluating its efficacy6). Measuring serologic antibody titer is widely used because of its simplicity7). In particular, the standardized hemagglutination inhibition (HI) test is widely used because of its reliability, sensitivity, and specificity in evaluating immunogenicity of influenza vaccines8).
Neutralizing antibody (NAb) is another key factor for evaluating host immunity against influenza, but measuring NAb takes longer than other tests and is difficult to standardize for evaluating the immunogenicity of influenza vaccine9-11). Recently, with the development of an improved microneutralization (MN) assay, NAb measurement has been increasingly adopted in several countries, but not in Korea12,13). Here, we tried conventional MN test to identify usefulness as an alternative or complementary test to the HI test. Though enzyme-linked immunosorbent assay (ELISA) based MN tests have become popularly used, we adopted conventional MN because we thought the classic methods were more valuable as the initial trial of MN test for evaluating the immunogenicity of influenza vaccine in Korea than ELISA based MN test.
Materials and methods
1. Patient population and materials
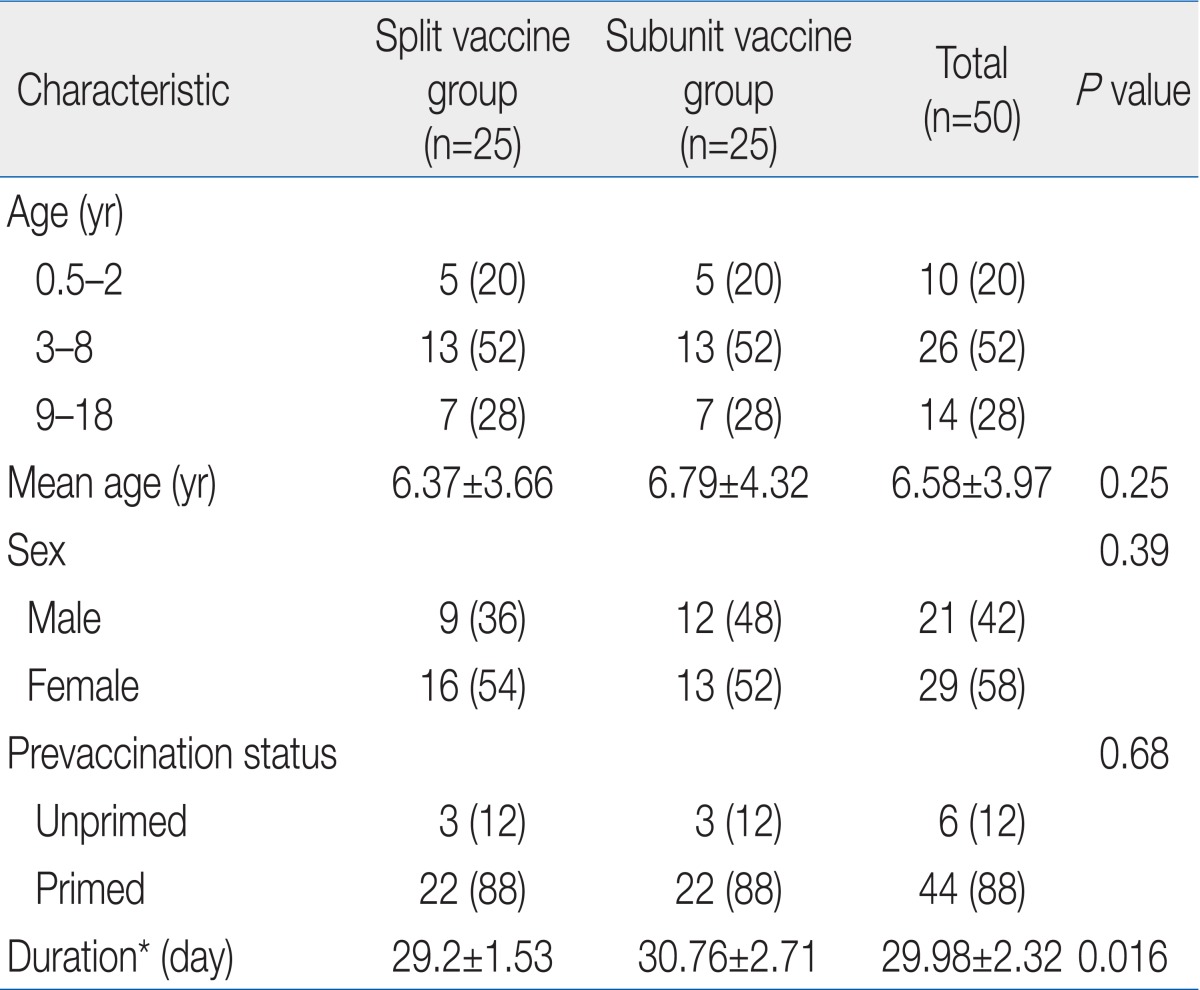
We randomly selected 50 paired samples (25 paired samples in 153 split vaccine group and 25 paired samples in 158 subunit vaccine group) among 303 pairs of prevaccination and 30 days postvaccination human serum samples for analysis by MN test. The study protocol was approved by the Institutional Review Board of Korea Cancer Center Hospital. The samples were taken during a previous clinical study that evaluated the immunogenicity of split and subunit influenza vaccines with HI test. The influenza virus strains used in this study were A/Brisbane/59/2007(H1N1) strain (IVR-148), A/Uruguay/716/2007(H3N2) strain (NYMCX-175C), and B/Florida/4/2006.
2. HI test and evaluation of immunogenicity
Chicken Red Blood Cells, 5%, were used for HI test. Seroprotection rate, seroconversion rate, and geometric mean titer (GMT) were used for evaluating immunogenicity of vaccines. HI antibody titer 1:40 was defined as antiinfluenza protective antibody titer. Seroconversion was defined as a change from a baseline titer of <1:10 to a postvaccination titer of ≥1:40 or a 4-fold or greater rise in titer in samples with an initial HI titer ≥1:10. Seroprotection was defined by titers of ≥1:40.
3. Madin-Darby canine kidney (MDCK) cell preparation
We calculated cell concentration after incubating cells on a 96 well plate with 100 µL of MDCK (ATCC, Manassas, VA, USA) cells in each well seeded in medium at 1.5×105 cells/mL. The plate was incubated for 24 hours at 37℃ and 5% CO2.
4. Virus titration
If there was plaque formation or cytopathogenic effect (CPE) in MDCK cells, we considered them to be infected by influenza virus.
We preferentially took 146 µL of virus culture medium and reserved it at 4℃ for a period after thawing. Culture medium containing trypsin, 100 µL, was added in the 96 wells. The reserved 146-µL virus culture medium was added in the wells of first row, and 46 µL of diluent was decanted serially in the next row. Afterwards, each well was prepared with culture medium attenuated gradually by 1/2 log10. After 48 hours incubation at 37℃ in a 5% CO2 humidified atmosphere, we evaluated viral plaque formation in MCDK cells by optical microscopy. In order to account for any variability in the test procedure, each sample was tested in duplicate. When two results were mismatched, retests were done till matched.
After culture media of the wells with plaques were removed, the MDCK cells were stained by crystal violet dye, and the number of wells forming plaques was counted. The 50% tissue culture infecting dose (TCID50) was calculated using the method of Reed and Muench, and 50-µL virus culture diluents containing 100 TCID50 virus were used in each well for MN test.
5. MN test and evaluation of immunogenicity
MDCK cells were added to each well of a 96 well plate. A diluent of 90 µL and 10 µL heat inactivated human sera was added to the first well and 2-fold serial dilutions were performed in an equal volume of diluent in the plate, and 50 µL of diluent containing 100 TCID50 of influenza virus was added to each well.
After a 48 to 120 hours incubation at 37℃ in a 5% CO2 humidified atmosphere, we examined viral plaque formation in the MCDK cells with optical microscopy. Culture fluid with and without plaques was removed from the 96 wells and stained with crystal violet dye.
NAb titer was established at the maximal diluted concentration inhibiting plaque formation. When plaque formation was not inhibited in 1:10 sera, we set the NAb titer to 1:5.
In this study, seroprotection rate, seroconversion rate, and GMT were used as the surrogate markers for evaluating immunogenicity of vaccines by MN test.
NAb titer 1:40 was defined as an antiinfluenza protective antibody titer. Seroconversion was defined as a change from a baseline titer <1:10 to a postvaccination titer ≥1:40 or a 4-fold or greater rise in titer in samples with an initial NAb titer ≥1:10. Seroprotection was defined as a titer of ≥1:40. MN test for H3N2, H1N1, and influenza B was duplicated for each serum sample.
6. Approval standard criteria for influenza vaccine
Because there are no standard approval criteria in Korea for immunogenicity of influenza vaccines, this study used the following standard authorization criteria from European Medicines Agency (EMEA): 1) seroconversion rate of NAb titer >40%, 2) NAb GMT fold increase >2.5, 3) percentage of subjects with NAb titer over 1:40 ≥70%.
7. Statistical analysis
All calculations were performed with SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). To test for correlation between HI and MN test, the correlation coefficient square, R2, was calculated for correlation analysis and the kappa index was calculated for evaluation the index of coincidence.
A chi-square test was used to compare the seroprotection rate and seroconversion rate between the split influenza vaccine group and the subunit influenza vaccine group. We analyzed the 95% confidence interval for GMT comparisons, and P<0.05 was considered statistically significant.
Results
Paired samples of 50 subjects (age, 6.0 months to 18.0 years; mean age, 6.58±3.97 years) were analyzed. There were no statistical differences in mean age, sex ratio, and prevaccination status between split vaccine group and subunit vaccine groups (Table 1).
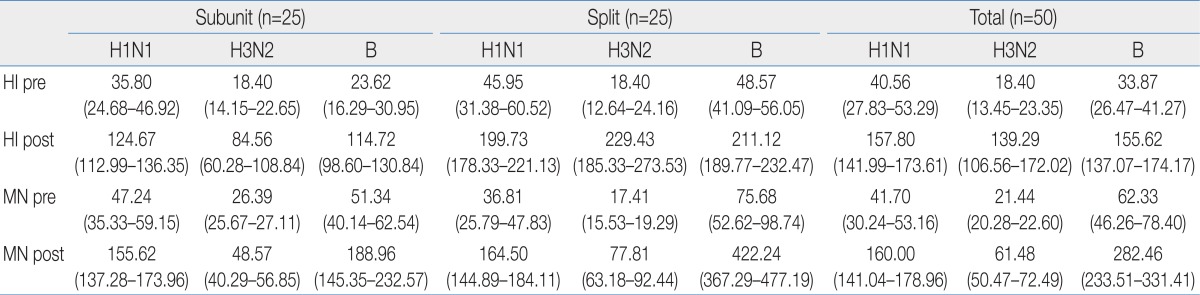
The linear correlation coefficient squares, R2, of MN and HI test for H1N1, H3N2, and influenza B were 0.70, 0.69, and 0.66, respectively, and we observed a high index of coincidence between the two tests (Fig. 1).
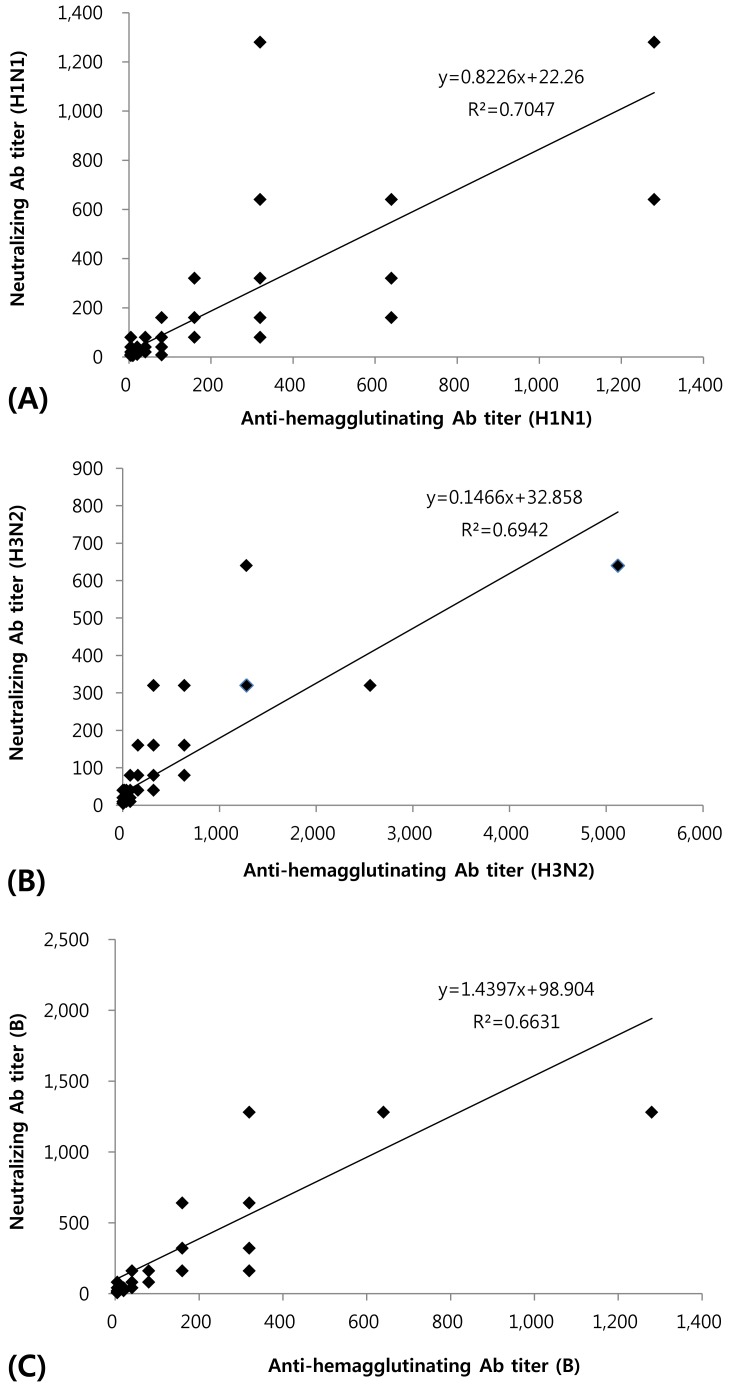
Relationship between hemagglutination inhibition and neutralization against A/Brisbane/59/2007 (H1N1) strain (A), A/Uruguay/716/2007 (H3N2) strain (B), and B/Florida/4/2006 (B) strain (C). Ab, antibody.
A 1:40 titer on HI test is widely accepted as an effective antiinfluenza antibody titer, but no standard value for MN test has been widely accepted. According to the results of this study, NAb titers of 1:40 rather than 1:80 were highly significantly coincident with HI test titers, as indicated by the higher kappa index (Table 2).
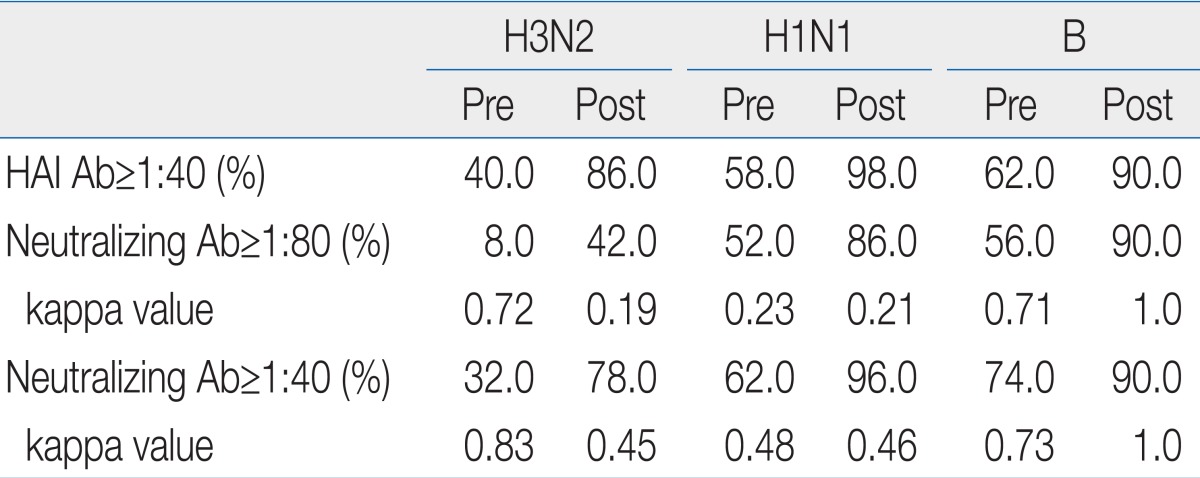
Comparison of the Hemagglutination Inhibition Test and Microneutralization Test for Evaluating the Immunogenicity of Influenza Vaccines
When we set a NAb titer of 1:40 for the antiinfluenza antibody titer, we observed 78.0%, 96.0%, and 90% seroprotection rates, all of which were greater than 70%, in the 30 day postvaccination specimens, compared with 32.0%, 62.0%, and 74.0% prevaccination rates for H3N2, H1N1, and influenza B, respectively.
The seroconversion rates of MN test were 42.0%, 42.0%, and 48.0% for H3N2, H1N1, and influenza B, and the GMTs were >2.5-fold (2.89, 5.04, and 4.29) (Fig. 2).

Immunogenicity of the 2008-2009 season influenza vaccine. (A) Seroprotection rate and geometric mean titer (GMT) of each influenza strain determined by the microneutralization test. (B) Seroconversion rate of each influenza strain determined by the microneutralization test.
We divided the subjects into 2 groups, an influenza subunit vaccine group and a split vaccine group, and evaluated immunogenicity of each group using MN test. We found no significant difference between the groups for seroprotection rate. However, there was significant difference in the H3N2 seroconversion rates between the 2 groups (Table 3). The H3N2 seroconversion rate of the split vaccine group was higher than that of the subunit vaccine group (46.7% vs. 25.7%, P=0.018). There were also significant differences between the 2 groups for NAb GMTs for H3N2 and influenza B (Table 4).
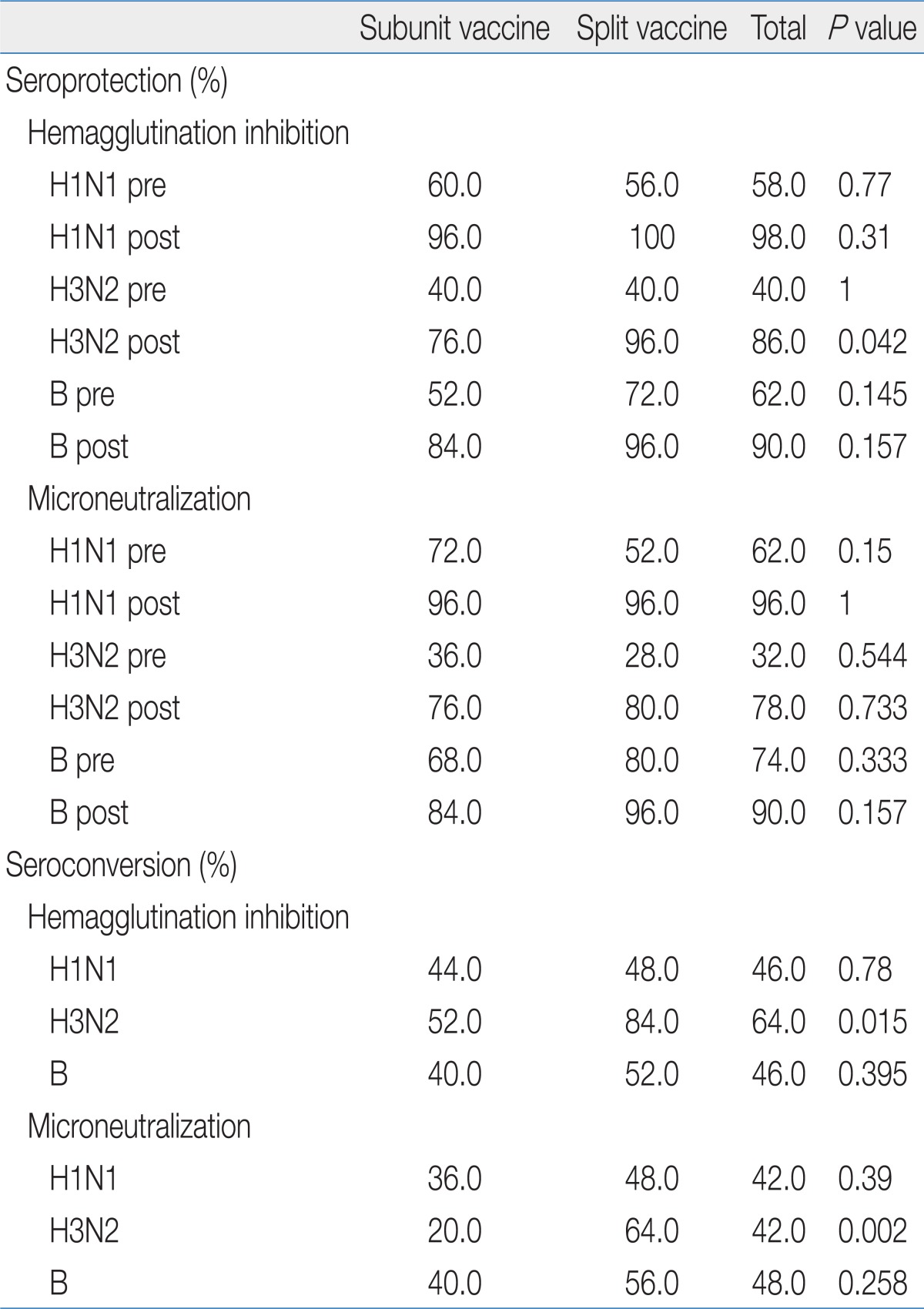
Seroprotection/Seroconversion Rates Determined by the Hemagglutination Inhibition Test and Microneutralization Test for Each Influenza Strain in Split and Subunit Vaccines
Discussion
The neutralization test (NT) is one of the most trusted and highly sensitive and specific methods currently employed for evaluating the immunogenicity of influenza vaccines. However, NT is a laborious and time-consuming procedure, making it less suitable for testing the large numbers of samples that are obtained in clinical trials.
The traditional method of measuring NAb activity is a plaque inhibition assay. The cytopathic effect of an influenza virus on infected MDCK cells is shown as a process of time; the influenza virus infected MDCK cells are detached from the cell layer because the adhesive strength between the cells and the surface adhesive strength are decreased, and plaque formation progresses at the site of detached cells. Conventional NT using the plaque inhibition assay is based on the tendency for plaque formation to be inhibited in proportion to the neutralization of influenza virus by NAbs in human sera. If there is plaque formation or CPEs on MDCK cells, the cells are regarded as infected by the influenza virus. The NAb titer is defined as maximal diluted concentration inhibiting a plaque14-16).
Many researchers have tried to develop a neutralization test that is effective for testing large numbers of samples in a short time. Okuno et al.9) reported a rapid focus reduction neutralization test for influenza viruses in a microtiter system. The test revealed that influenza virus infected MDCK cells can be stained with the peroxidase-antiperoxidase complex staining technique, and the number of influenza virus infected MDCK cells is decreased to some degree by neutralizing antibodies in human sera. This study described a neutralization test, compared its results with those obtained by other methods, examined the correlation of MN titers with other test titers, and reported shorter times than the conventional plaque reduction assay method9). Other studies have also reported that the MN enzyme immunoassay can acquire results in a shorter time and from smaller serum samples. MN enzyme immunoassay identifies the amount of influenza viral NP protein by fixing the infected cells after overnight incubation12).
Recently, MN tests and HI tests for A/New Caledonia/20/1999, A/Solomon Islands/3/2006, A/Brisbane/59/2007, and A/California/04/2009 strains were performed by the Centers for Disease Control and Prevention (CDC) for 28 children and 30 adults according to standard MN and HI protocols. Although the estimated correlation between HI and MN titers was high (r=0.82) for the seasonal vaccine strains, the MN assay generally yielded higher titers and detected more seroconversions to A/California/04/2009 than the HI assay. A linear regression model was used to predict the MN titer for seasonal influenza A (H1N1) viruses that corresponded to an HI titer of 40 and to measure titer achievement against the seasonal vaccine strain and the novel influenza A (H1N1) virus. In the pediatric population, an HI titer of 40 corresponded to an MN titer of 40, whereas in the adult population the corresponding MN titer was ≥160. Our study in Korean children under 18 years was based on the CDC report, using the MN titer of 1:40 for the seroprotection antibody titer against influenza infection17).
We examined NAb titers using the conventional MN. We observed a high index of coincidence between this MN test and the HI test. This study is the first in Korea to evaluate the immunogenicity of influenza vaccines by MN test.
In our study, the immunogenicity of 2008-2009 seasonal influenza vaccines was evaluated with MN test, and the seroprotection rate and seroconversion rate of the vaccine fulfilled all the criteria for influenza vaccine in EMEA.
Comparing the HI and MN tests for influenza A-H1N1, H3N2, and B, we found similarity between the tests for influenza A-H1N1, but the HI test showed higher immunogenicity for H3N2 than the MN test, and MN test showed higher immunogenicity for influenza B than the HI test. Recently, the MN test was reported to yield higher titers and detect more seroconversions to A/California/04/2009 than the HI test.
The sensitivity and specificity of the NT for evaluating the immunogenicity of influenza vaccines have been found superior to those of the HI test, because the NT can evaluate lower antibody titers than the HI test. However, it is difficult to standardize the NT, and it is difficult to compare the NT to the HI test directly.
The HI test was less sensitive for influenza B than for influenza A, and was shown to have lower immunogenicity for influenza B than influenza A18). MN test was sensitive for influenza B and influenza A, and we concluded that MN test is a better method to evaluate human immunity against influenza B viruses.
Two types of inactivated influenza vaccine, a subunit vaccine and a split vaccine, are currently widely used. The subunit vaccine has purified influenza virus hemagglutinin and neuraminidase antigens, and the split vaccine has purified influenza virus hemagglutinin, neuraminidase antigens, and internal proteins of influenza virus. According to meta-analysis of the immunogenicity of influenza vaccines, there are no differences between the 2 types of vaccine, although some studies have reported differences between the vaccine types19).
In our study, the split vaccine showed somewhat better immunogenicity in MN and HI tests. However, the result is in need of additional investigation, especially since there was a large increase of the GMT against influenza B in postvaccination samples in the split vaccine group. We thought this finding resulted from a higher basal level of GMT against influenza B in the split vaccine group, which implies that there were many more subjects primed with previous influenza B in the split vaccine group. The antigen exposure after priming resulted in rapid production of antibodies20-22).
For this reason, a small difference of the basal GMT against a virus produced a large difference in postvaccine GMT, and we could not conclude that there was better immunogenicity of the split vaccine than the subunit vaccine against influenza B. Subsequently, the result will need additional investigation.
Because there are no standard approval criteria in Korea for immunogenicity of an influenza vaccine, the EMEA criteria have been informally adopted. The standard authorization criteria of the EMEA for immunogenicity of influenza vaccine include HI and single radial hemolysis techniques. The NAb test results can be evaluated with the authorization criteria from EMEA.
In our study, the immunogenicity of the seasonal 2008-2009 influenza vaccines used in Korea was evaluated using a MN test, and the MN test showed results satisfying standard authorization criteria of the EMEA: seroconversion rate of NAb titer >40%; NAb GMT fold increase >2.5; and percentage of subjects with NAb titer over 1:40 ≥70%. As a result, 2 types of seasonal 2008-2009 influenza vaccines used in Korea had an allowable immunogenicity.
In conclusion, we achieved a desirable result in evaluation of the immunogenicity of 2008-2009 seasonal influenza vaccines using a MN test. The linear correlation coefficient between MN and HI test for influenza vaccines were determined, and the MN test was as satisfactory as the HI test for evaluating the immunogenicity of influenza vaccines. Therefore, we recommend the MN test for an alternative or complementary test to HI test to evaluate the immunogenicity of influenza vaccines.
Acknowledgment
This study was accomplished with the financial support of the KFDA (Korea Food and Drug Administration, 09122KFDA426).