Clinical features and surgical outcomes of complete transposition of the great arteries
Article information
Abstract
Purpose
This single-center study aimed to assess the clinical features and surgical approaches and outcomes of complete transposition of the great arteries (TGA).
Methods
TGA patients who had undergone surgical correction at the Kyungpook National University Hospital from January 2000 to December 2010, were retrospectively evaluated for patient characteristics, clinical manifestation, preoperative management, intraoperative findings, postoperative progress, and follow-up status.
Results
Twenty-eight patients (17 boys and 11 girls, mean age=10.6±21.5 days) were included and were categorized as follows: group I, TGA with intact ventricular septum (n=13); group II, TGA with ventricular septal defect (VSD, n=12); and group III, TGA/VSD with pulmonary stenosis (n=3). Group I underwent the most intensive preoperative management (balloon atrial septostomy and prostaglandin E1 medication). Group II showed the highest incidence of heart failure (P<0.05). Usual and unusual coronary anatomy patterns were observed in 20 (71%) and 8 patients, respectively. Arterial and half-turned truncal switch operations were performed in 25 and 3 patients (Group III), respectively. Postoperative complications included cardiac arrhythmias (8 patients), central nervous system complications (3 patients), acute renal failure (1 patient), infections (3 patients), and cardiac tamponade (1 patient), and no statistically significant difference was observed between the groups. Group II showed the mildest aortic regurgitation on follow-up echocardiograms (P<0.05). One patient underwent reoperation, and 1 died. The overall mortality rate was 4%.
Conclusion
Our study showed favorable results in all the groups and no significant difference in postoperative complication, reoperation, and mortality among the groups. However, our results were inadequate to evaluate the risk factors for reoperation and mortality owing to the small number of patients and short follow-up duration.
Introduction
Transposition of the great arteries (TGA) is a congenital heart anomaly with 0.45 cases per 1,000 live births, and the arterial switch operation (ASO) has become the treatment of choice for surgical correction of complete TGA. Since the first successful ASO was reported by Jatene et al.1) in 1975, the mortality and morbidity have been reduced by variations in surgical techniques, such as that described by Lecompte et al.2), which reduce the likelihood of pulmonary outflow obstruction. In contrast to the atrial switch procedures (Mustard and Senning operation), the ASO has the advantage of the maintenance of sinus rhythm, utilization of the left ventricle as the systemic ventricle and the mitral valve as the systemic atrioventricular valve3). For patients with TGA, ventricular septal defect (VSD) and left ventricular outflow tract obstruction/pulmonary stenosis (PS), the Rastelli operation, REV procedure and modified Nikaidoh procedure have been developed, and these procedures were observed favorable with long-term result and survival rate4,5). Recently, many centers in South Korea reported satisfactory short-term and long-term postoperative results of complete TGA6,7).
The purpose of this study is to assess the clinical features and surgical approaches, as well as the results of complete TGA in a single center.
Materials and methods
1. Patients
All patients who were diagnosed as complete TGA and underwent open heart surgery at Kyungpook National University Hospital between January 2000 and December 2010 were included. We retrospectively reviewed the medical records, which included patients' characteristics, clinical manifestation, preoperative management, operation report, postoperative progress and follow-up status. The patients were divided into three groups according to combined anomalies, and we analyzed the collected data between the groups.
2. Statistics
PASW ver. 18.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. All values are expressed as the mean±standard deviation. The between-group differences were analyzed by the unpaired Student's t-test or chi-square analysis. P values of <0.05 were considered significant.
Results
1. Demographics and cardiac anatomy
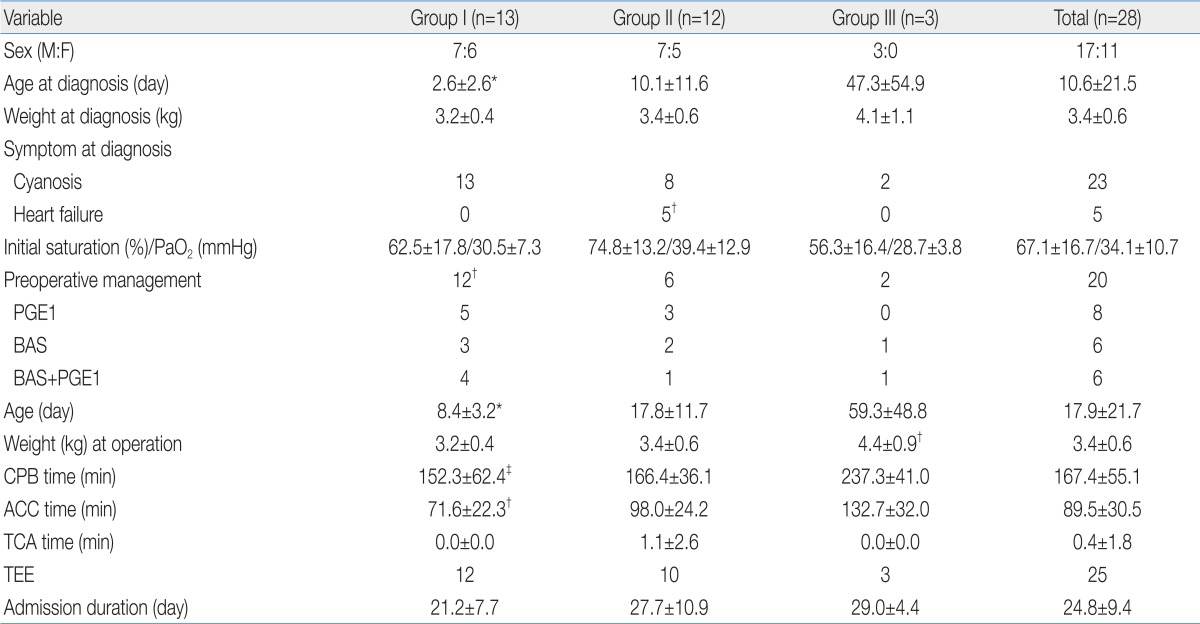
There were 28 patients (17 boys and 11 girls) with the mean age at diagnosis of 10.6±21.5 days (range, 1 to 108 days), and the mean body weight at diagnosis of 3.4±0.6 kg (range, 2.4 to 5.3 kg) (Table 1).
According to combined anomalies diagnosed by 2D-echocardiogram, the patients were divided into three groups; group I (TGA/intact ventricular septum) with 13 patients (46%), group II (TGA/VSD) with 12 patients (43%) and group III (TGA/VSD with PS) with 3 patients (11%).
All 28 patients had atrial septal defect (ASD) and 24 had patent ductus arteriosus (PDA), with or without VSD. Among the group II patients, 2 had atrioventricular septal defect, 1 had coactation of aorta (CoA) and 1 had interrupted aortic arch (IAA) type B. Two patients of the group III had valvular PS and 1 had subpulmonic stenosis.
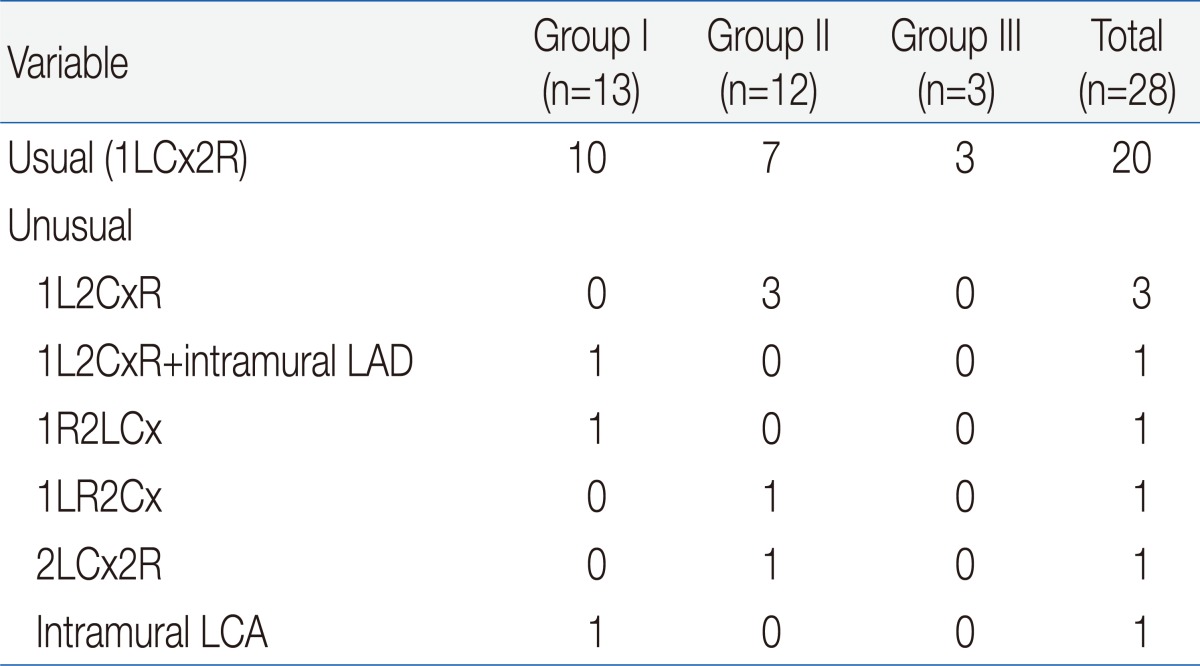
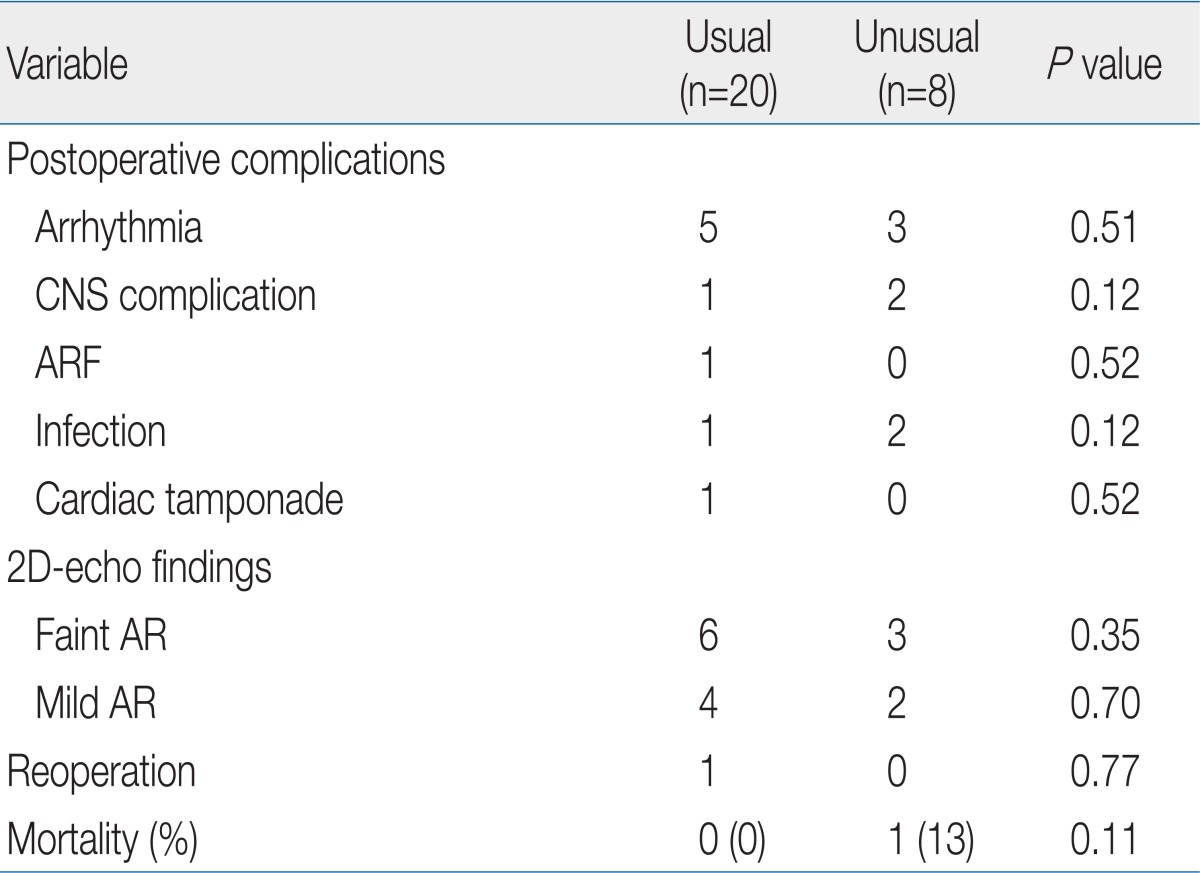
To understand coronary artery anatomy, we used 2D-echocardiogram in all patients and cardiac catheterization in 12 patients. Twenty patients (71%) had usual coronary pattern (1LCx2R) and 8 patients (29%) had unusual pattern: left circumflex artery (LCx) from right coronary artery (RCA) (1L2CxR) in 3, LCx from RCA (1L2CxR) with intramural left anterior descending in 1, inverted origins (1R2LCx) in 1, inverted RCA and LCx (1LR2Cx) in 1, single RCA (2LCx2R) in 1, and intramural left coronary artery in 1. There was no significant difference in the coronary artery patterns between the groups (Table 2).
2. Clinical manifestation and preoperative management
The initial clinical presentation was cyanosis in 19, heart murmur only in 5 and both cyanosis with heart murmur in 4 patients. Five patients showed heart failure symptoms (e.g., tachypnea and poor feeding), and were treated by diuretics with/without digitalization. These patients were all included in group II, and it was shown to have significantly higher incidence than the other groups (Table 1).
For preoperative management, balloon atrial septostomy (BAS) and prostaglandin E1 (PGE1) continuous intravenous infusion was performed in 6 patients, BAS only in 6 and PGE1 medication only in 8. Group I performed significantly more cases of preoperative management than the other groups (P<0.05), but there was no significant difference in the initial pulse oxymeter saturation and arterial oxygen content between the groups (Table 1). Eight patients with no preoperative management showed higher pulse oxymeter saturation (SpO2, 75.1±14.7%) and arterial oxygen content (PaO2, 39.0±8.6 mmHg) than the patients who needed both BAS and PGE1 (SpO2, 63.9±16.8%; PaO2, 32.2±11.0 mmHg), but there was no statistical difference.
3. Operation and postoperative results
The mean age at operation was 17.9±21.7 days (range, 3 to 114 days) and the mean body weight at operation was 3.4±0.6 kg (range, 2.4 to 5.2 kg) (Table 1). Patients of group I and II (n=25, 89%) underwent ASO with total correction of VSD, ASD, PDA, PA branch stenosis and CoA/IAA, and half turned truncal switch operation was performed in group III patients (n=3, 11%). In 3 patients with unusual coronary artery pattern, the pericardial hood trap door technique was performed during a coronary transfer procedure.
Intraoperative trans-esophageal echocardiogram was taken in 25 patients (89%), and the mean cardiopulmonary bypass (CPB) time was 167.4±55.1 minutes (group I significantly shorter than group III), aortic cross clamp time was 89.5±30.5 minutes (group I significantly shorter than the other groups) and total cardiac arrest time was 0.4±1.8 minutes. The mean hospital stay duration was 24.8±9.4 days (range, 5 to 47 days) (Table 1).
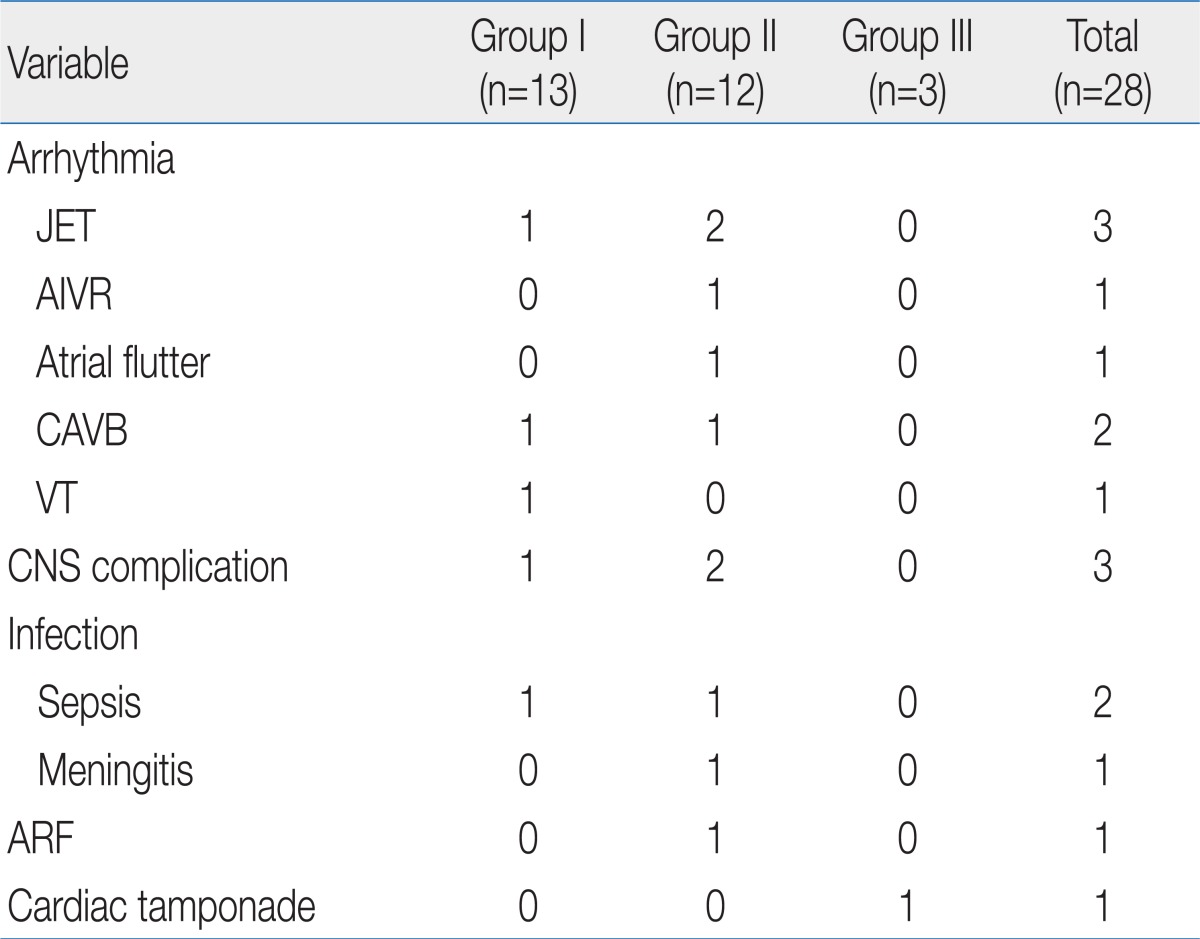
The early postoperative complication included cardiac arrhythmia, central nerve system (CNS) complication, acute renal failure, infection and cardiac tamponade, which were found in 16 cases (14 patients, 50%). Cardiac arrhythmia was found in 8 cases; 3 junctional ectopic tachycardia, 1 accelerated idioventricular rhythm, 1 atrial flutter, 2 complete atrioventricular block (CAVB) and 1 ventricular tachycardia (VT). The patient with VT (in group I) was undertaken several times direct-current (DC) cardioversion and amiodarone infusion, but finally expired 12 hours after the operation. One case of CAVB in group I was found immediately after open heart surgery at the operating room, treated with pacing and recovered with normal sinus rhythm, 5 hours later. The other patient with CAVB in group II also showed acute renal failure 2 days after the operation and was treated with 13 days pacing and 5 days PD. CNS complication was intraventricular and intracranial hemorrhage with/without hydrocephalus in 3 cases, and all of them showed seizures, and were treated with anti-epileptic drugs. Brain lesions were resolved spontaneously in two cases, but one case was with severe hydrocephalus and needed ventricular peritoneal shunt and this patient still has it. Infection was observed in 2 cases of sepsis and 1 case of meningitis, and they were treated with antibiotics. Acute renal failure was developed in 1 case, in group II, who recovered after peritoneal dialysis for 5 days. One case in group III with bradycardia and hypotension, after an open heart surgery, was diagnosed with cardiac tamponade and underwent emergent re-operation. The prevalence of immediate postoperative complication was not shown with statistically significant difference between the groups (Table 3).
There was 1 case of death during the immediate postoperative period, so the early mortality rate was 4%.
4. Follow-up status
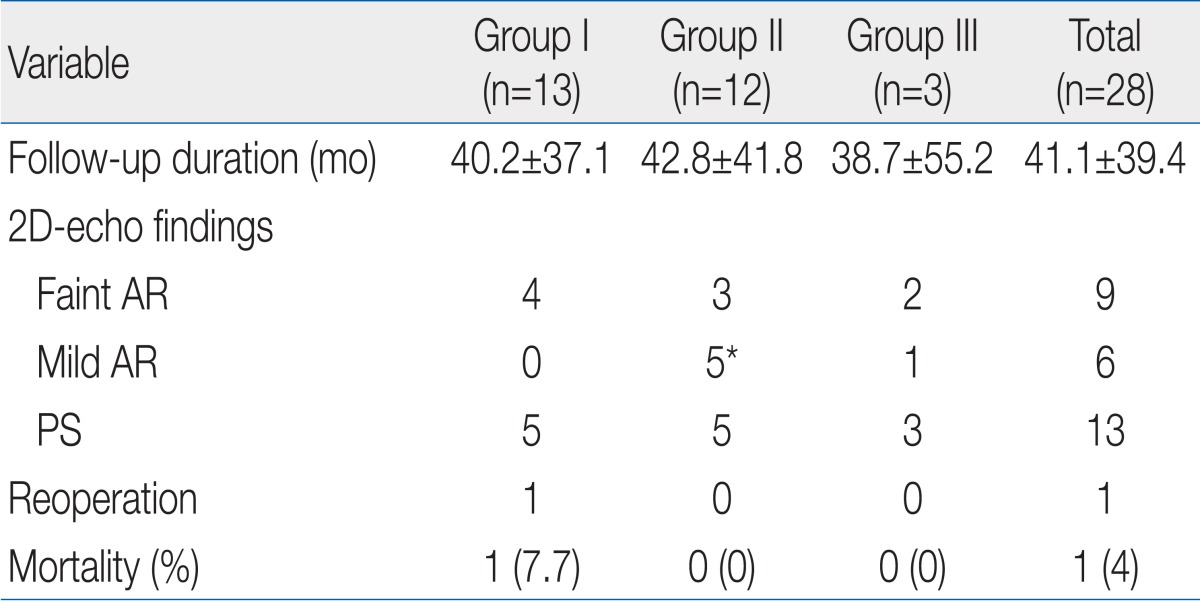
The mean duration of follow-up was 41.1±39.4 months (range, 1 to 130 months) and the follow-up 2D-echocardiogram showed faint aortic regurgitation (AR) in 9 patients, mild AR in 6, faint to mild pulmonary regurgitation in 5, mild PS in 13, and no moderate to severe AR. Patients in group II showed significantly more incidence of mild AR than the other groups (Table 4).
There was 1 case of reoperation (in group I), who with severe pulmonary artery (PA) branch stenosis underwent PA angioplasty 43 months after ASO. There was no late mortality, so the overall mortality rate was 4%. Further, there was no significant difference in the postoperative complication and long-term follow-up data between the coronary artery patterns (Table 5).
Discussion
Our study showed favorable surgical results of complete TGA in a single center. The early mortality in this study was 4% (1/28), and it was similar to the other published large studies, where the mortality rate of ASO has been reported between 1.6 and 11%8-12). In addition, like the other reports13,14), half-turned truncal switch operation showed a good result for the complete TGA with VSD and PS (the mortality rate in group III, 0%).
The known risk factors for the mortality and morbidity for ASO in complete TGA were combined with anatomical defects, unusual coronary artery anatomy, prolonged CPB time, preterm birth and low body weight at the time of the operation8,10,15,16). Although the presence of a VSD or aortic arch obstruction in complete TGA has been described as a significant risk factor10,17), we showed favorable outcome in spite of different types of TGA anatomy.
In the previous studies, it has been found that patients with unusual coronary patterns have a significantly increased mortality risk18-20). Pasquali et al.19) reported that the patients with any variant coronary pattern had an almost twofold risk of hospital mortality. They found that the highest risk patterns were an origin of all three coronary arteries from a single sinus and an intramural course of the coronary arteries with a threefold and greater than six fold increased mortality risk, respectively. In our study, there was no significant difference in the postoperative complication and mortality between the coronary artery patterns. Further, among the 8 patients with unusual coronary artery patterns, one patient with intramural LCA expired due to postoperative uncontrolled VT, and the other 7 patients survived without any coronary events. This may be due to coronary artery transfer techniques. The direct re-implantation technique does not always provide the exact coronary geometry, especially in cases with abnormal coronary arterial patterns. Therefore, pericardial hood trap door technique is helpful for the maintenance of the exact coronary geometry21).
CPB time greater than 150 minutes was an independent predictor of both hospital and intermediate-term mortality of ASO15). CPB has well-known time-dependent deleterious effects, including a whole-body inflammatory response, metabolic changes, and fluid and electrolyte imbalance22). In addition to these negative influences, prolonged CPB may also be a surrogate for other factors, such as complexity of anatomy and operation. In our study, although the mean CPB time was greater than 150 minutes, we showed a favorable outcome. But we could not evaluate, statistically, the significance between CPB time and mortality because of the small number of patients.
Another risk factor for increased mortality and morbidity is a low birth weight and prematurity8,15). Due to the immaturity of organ systems, these patients are exposed to a specific risk resulting from noxious effects of extracorporeal circulation, especially on the central nervous system23,24). Prandstetter et al.16) reported that the body weight, less than 2.5 kg at the time of the operation, was the only risk factor for increased hospital mortality. As surgical reasons for the adverse outcome of patients could not be identified, they concluded that immaturity of organ systems is the main reason for the increased risk of mortality and morbidity in this group. In that study, those who died late, after the surgery from grade IV postoperative cerebral bleeding, belonged to a low birth weight group. We found 3 cases of postoperative CNS hemorrhage with prevalence of 11% (3/28), but all patients were full term babies. And we also could not evaluate the statistical significance between the body weight at the time of the operation and mortality.
The most common reason for reoperation after ASO is PS, especially, supravalvular PS, with a recent reported prevalence of 7 to 21%6,25-27). The PS developed in various mechanisms, but mainly, inadequate somatic growth of the PA, which is localized at the suture line that causes a circumferential narrowing and flattening of the reconstructed right ventricular outflow tract. Other possible mechanisms may be the presence of ductal tissue, which may cause the so called 'coarctation' of the left PA and the 'bowstring' of the left PA over the aorta after the Lecompte procedure, causing a significant flow asymmetry27). The incidence of mild PS, in our study, is 46% (13/28) and one of them was supravalvular PS. Only one patient with severe PA branch stenosis in group I needed PA angioplasty after 43 months.
The AR is a frequently observed valve complication after ASO, and the incidence and the degree of AR progresses over time, but reoperation for AR was less common than that of PS6,25,28). The development of AR is related to more advanced age at the time of ASO, previous PA banding, use of trap-door coronary technique, size-discrepancies between the aorta and PA, AR at discharge, and a rapid development of aortic root dilatation6,28). In our study, faint to mild AR was fond in 54% (15/28) and the incidence of mild AR was significantly higher in group II, but there was no case of reoperation due to AR. The higher incidence of AR in group II is probably related to an increased preoperative flow across the anatomic pulmonary valve25), and it is the same reason that patients of group II showed more heart failure symptoms.
Our study demonstrated an overall favorable surgical outcome in complete TGA during hospitalization and intermediate-term follow-up. However, our study was performed retrospectively in a single center with a small number of patients and insufficient follow-up duration. As such, there was limited result to evaluate the risk factor for the reoperation and long-term mortality. Therefore, further studies will be needed to assess the long-term complications of complete TGA, such as PS and AR.