Happle-Tinschert syndrome in an infant: clinical, radiologic and genetic correlation
Article information
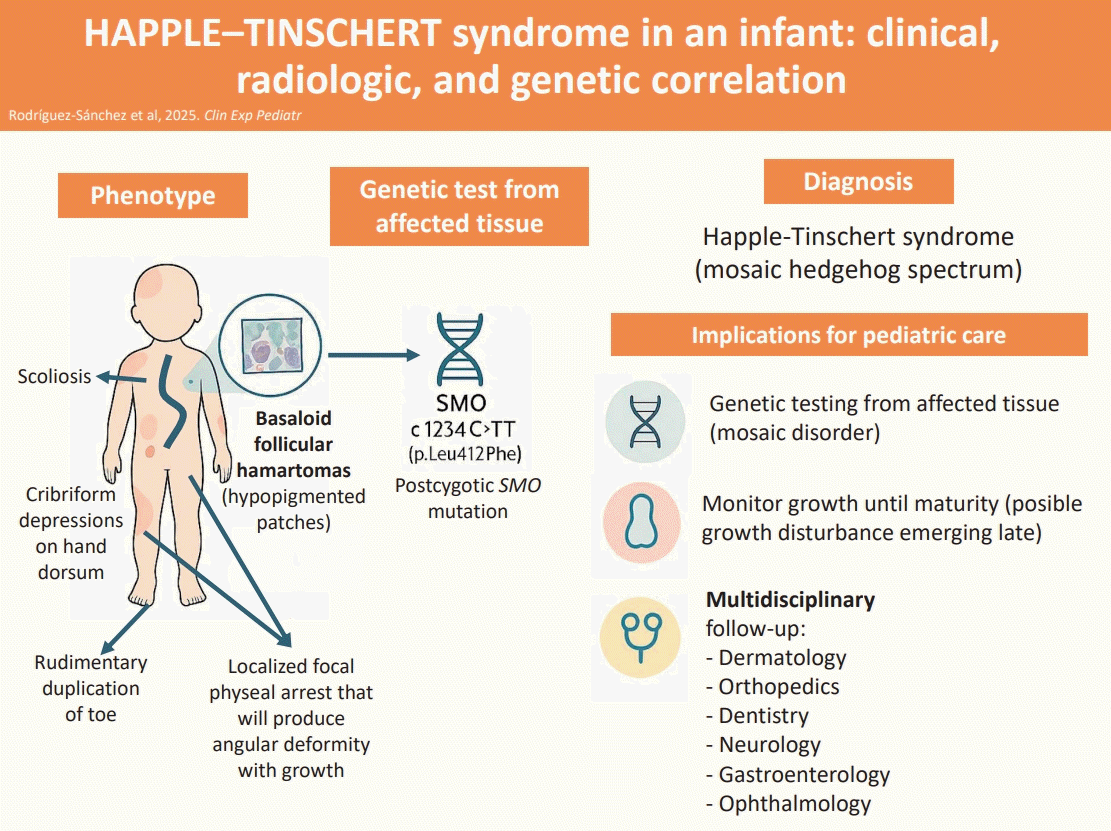
Graphical abstract. An infant with Happle-Tinschert syndrome presented with segmental cutaneous findings—blaschkoid hypopigmented patches consistent with basaloid follicular hamartomas and cribriform depressions—and skeletal involvement with scoliosis and asymmetric physeal bony bridges. Genetic testing of affected skin revealed a mosaic SMO c.1234C>T mutation, establishing the diagnosis of a mosaic hedgehog-spectrum disorder, specifically Happle-Tinschert syndrome, based on the clinical phenotype. Affected patients should be monitored through a multidisciplinary approach until skeletal maturity is achieved.
Happle-Tinschert syndrome (HTS) is a rare mosaic disorder characterized by segmental basaloid follicular hamartomas (BFH) and variable multisystem involvement, including skeletal, dental, and occasional neurologic abnormalities [1-5]. Although cutaneous findings often prompt initial evaluation, the condition is clinically relevant to pediatricians because growth-plate abnormalities can evolve silently and cause progressive deformities throughout childhood [3,4]. Early recognition, targeted genetic testing of affected tissue, and multidisciplinary follow-up are essential for adequate care.
HTS belongs to the mosaic hedgehog-pathway spectrum, which also includes Curry-Jones syndrome and segmental basal cell nevus syndrome. These entities arise from postzygotic mutations in hedgehog-signaling genes—most often SMO or PTCH1—and represent a phenotypic continuum depending on mutation timing, tissue distribution, and variant allele fraction [4]. Understanding this mosaic nature helps explain the patchy presentation and the potential for progressive findings.
A 3-month-old full-term girl with normal perinatal history and normal general health presented with whorled hypopigmented patches on the trunk, left upper arm, and left thigh following Blaschko lines (Fig. 1A). Over some lesions, millimetric white papules resembling milia were noted (Fig. 1B).
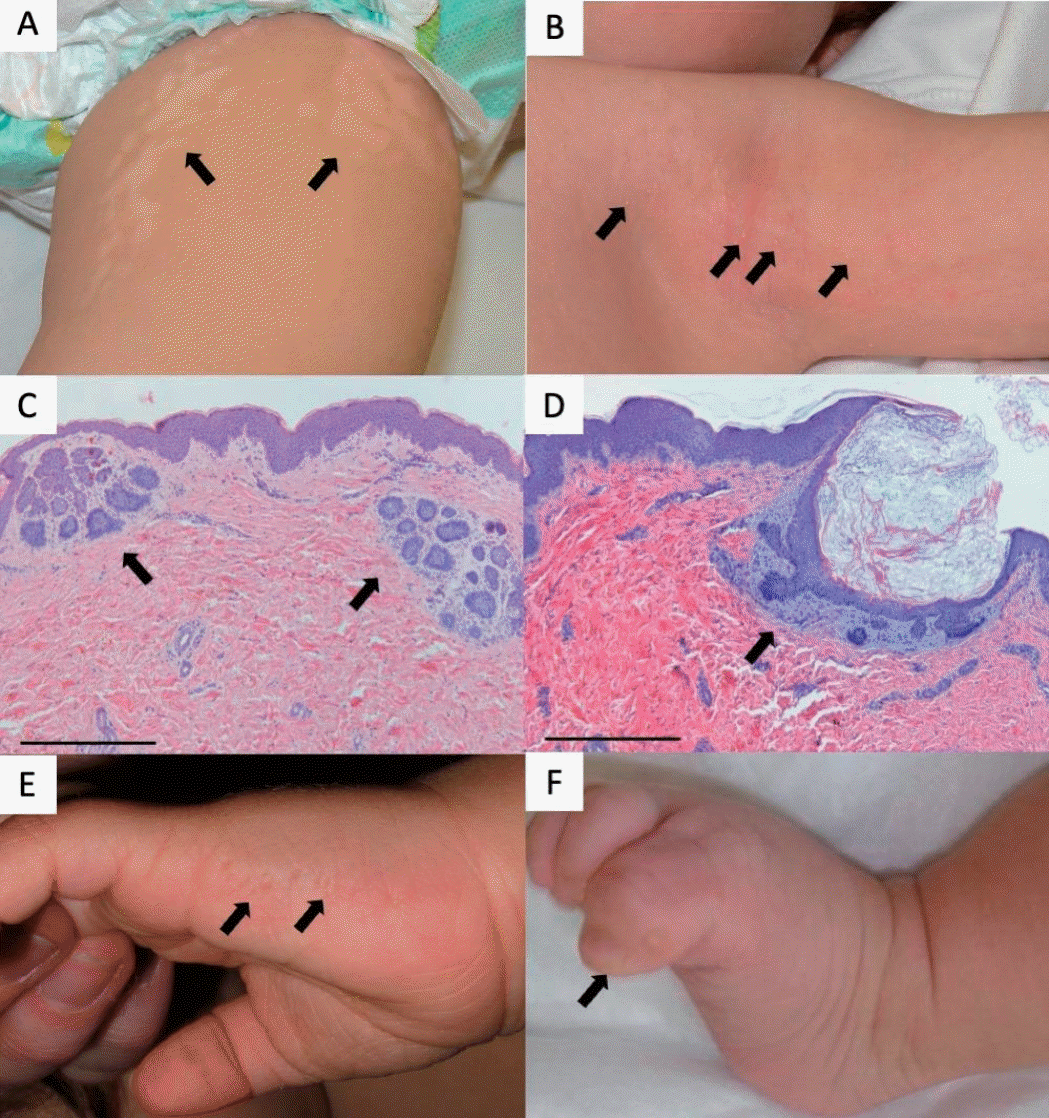
Clinical and histological features. (A) Hypopigmented patches with blaschkoid distribution on the left thigh (arrows). (B) Milia-like papules over a hypopigmented patch in the left axilla (arrows). (C) Anastomosing strands of basaloid cells consistent with basaloid follicular hamartoma (arrows) (hematoxylin & eosin [H&E], ×4, scale bar=500 μm). (D) Epidermoid cyst with basaloid anastomosing strands (arrow) (H&E, ×4, scale bar=500 μm). (E) Cribriform depressions on the medial dorsum of the left hand (arrows). (F) Rudimentary duplication of the left fifth toe (arrow).
Histopathology from a hypopigmented area revealed multiple anastomosing strands of basaloid cells in a loose stroma, consistent with basaloid follicular hamartoma (Fig. 1C). A papule corresponded to an epidermoid cyst associated with anastomosing basaloid strands (Fig. 1D).
She also had cribriform depressions on the medial dorsum of the left hand (Fig. 1E) and a rudimentary duplication of the left fifth toe (Fig. 1F). Neurologic development, feeding, sleep, and growth milestones were age-appropriate, and no systemic symptoms were reported during infancy.
As the patient grew, scoliosis became evident, and she developed right-sided genu varum. Radiographs demonstrated asymmetric physeal bone bridges across the medial distal femoral and proximal tibial physes on the right, with additional bridges across the left tibial physis and the left femoral head physis; the latter was associated with coxa breva (Figs. 2A and B). These bridges produced focal growth arrest rather than diffuse physeal involvement.
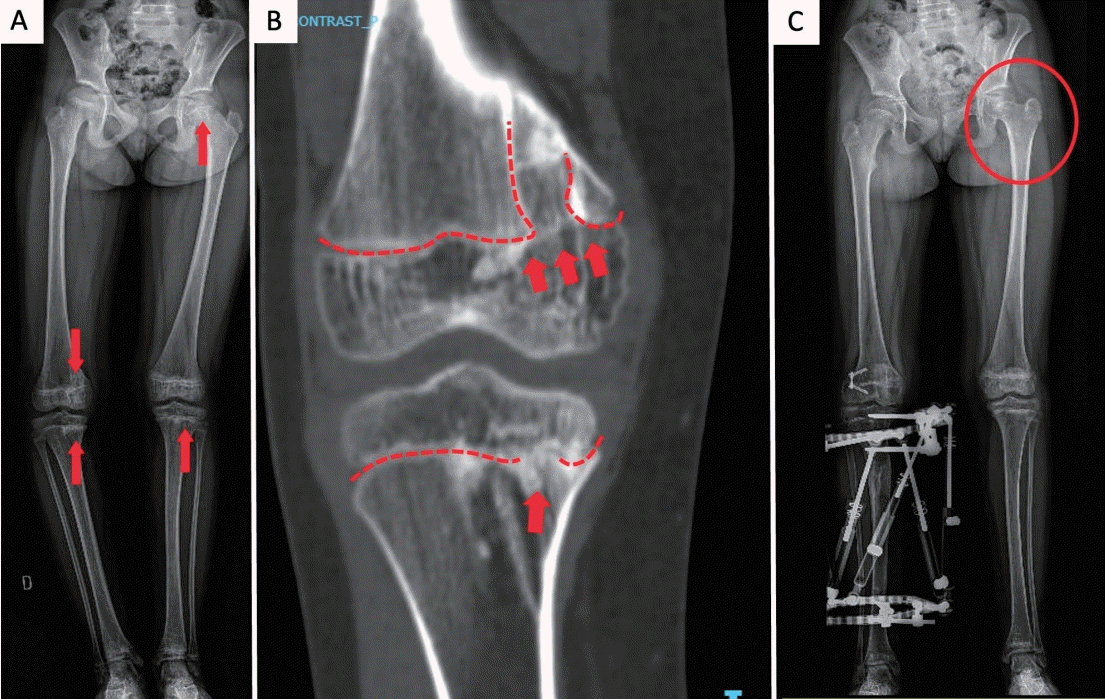
Radiographic features and management. (A) Lower-limb radiograph showing osseous bridges across the right medial distal femoral and proximal tibial physes (genu varum), left tibial physis, and left femoral head physis (coxa breva) (arrows). (B) Detail of medial femoral/tibial bridges; the dashed lines indicate physes interrupted by bridges (arrows). (C) Postoperative radiograph showing lateral distal femoral hemiepiphysiodesis and tibial external fixator for medial distraction osteogenesis; the circle marks the left coxa breva.
Orthopedic correction comprised lateral distal femoral hemiepiphysiodesis to restrain lateral growth and tibial distraction osteogenesis with an external fixator to restore medial length and alignment, achieving correction of genu varum and limb-length equality (Fig. 2C).
A brain magnetic resonance imaging, ophthalmologic evaluation, and abdominal examination were normal. No gastrointestinal or dental anomalies were detected.
Targeted sequencing of affected skin identified a mosaic NM_005631.5(SMO):c.1234C>T (p.Leu412Phe) variant with a 23% allele fraction, confirming a hedgehog-pathway mosaic disorder most consistent with HTS [4,6-8]. The variant was absent in blood, consistent with postzygotic mosaicism.
HTS is a mosaic disorder driven by postzygotic mutations in hedgehog-signaling genes. The SMO p.Leu412Phe variant identified in this case has been repeatedly associated with BFH and with phenotypes across the mosaic hedgehog spectrum. Its mosaic distribution likely accounts for the patient’s asymmetric skeletal involvement [3,4,8,9].
Skeletal abnormalities described in HTS include limb-length discrepancies, scoliosis, and digital anomalies, usually attributed to global physeal disturbance [3,4,8]. In contrast, our patient exhibited localized physeal bridges at multiple independent sites, producing a combination of angular deformity (right genu varum) and regional growth disturbance (coxa breva). This supports the concept that mosaic hedgehog-pathway disorders can produce focal physeal tethering, even when cutaneous involvement appears modest.
For pediatricians, the key implications are:
• Cutaneous clues (linear or patchy lesions along Blaschko lines) should prompt evaluation for underlying mosaicism.
• Blood tests may miss pathogenic variants; affected skin is the preferred tissue for genetic confirmation.
• Orthopedic complications may emerge progressively and require surveillance until skeletal maturity.
• Multidisciplinary care (dermatology, orthopedics, neurology, gastroenterology, dentistry, and ophthalmology) improves outcomes and family counseling.
BFH, although benign, are important diagnostic markers. Clinically, they may present as hypo- or hyperpigmented patches with comedo-like plugs, while histologically they show branching cords of basaloid cells with possible follicular differentiation. Stable lesions can be monitored in primary care; evolving or symptomatic lesions merit dermatology referral [3,4,8].
This case contributes to the expanding characterization of HTS by documenting asymmetric, focal physeal bridges as a mechanism of progressive deformity, reinforcing the need for long-term orthopedic surveillance.
This infant with HTS demonstrated classic cutaneous findings, focal skeletal involvement, and a mosaic SMO p.Leu412Phe variant detectable only in affected skin. Awareness of HTS and other mosaic hedgehog-pathway disorders is essential in pediatrics, as early diagnosis enables timely monitoring of growth-plate abnormalities and coordination of multidisciplinary care.
Written informed consent for publication of the clinical details and images was obtained from the patient’s parents.
Question
Which finding best explains the unilateral genu varum in this patient?
A. Generalized rickets
B. Asymmetric physeal bone bridges causing focal growth arrest
C. Neuromuscular imbalance due to scoliosis
D. Diffuse metaphyseal dysplasia
Answer: B
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: BRS, FJNC, JMNG, MDLPA, LZD, LJB, JSG, FAL, MCD; Data curation: BRS, FJNC, JSG, FAL, MCD; Formal analysis: BRS, LZD, JSG, FAL, MCD; Methodology: BRS, FJNC, JMNG, MDLPA, LZD, LJB, JSG, FAL, MCD; Project administration: BRS; Writing - original draft: BRS, FJNC, JMNG, MDLPA; Writing - review & editing: LZD, JSG, FAL, MCD