Allogeneic stem-cell transplantation following chimeric antigen receptor T-cell therapy for treatment of relapsed/refractory hematologic malignancy in children and young adults: a systematic review and meta-analysis
Article information
Abstract
Background
Allogeneic stem cell transplantation (allo-SCT) and chimeric antigen receptor (CAR) T-cell therapy offer potential complementary benefits.
Purpose
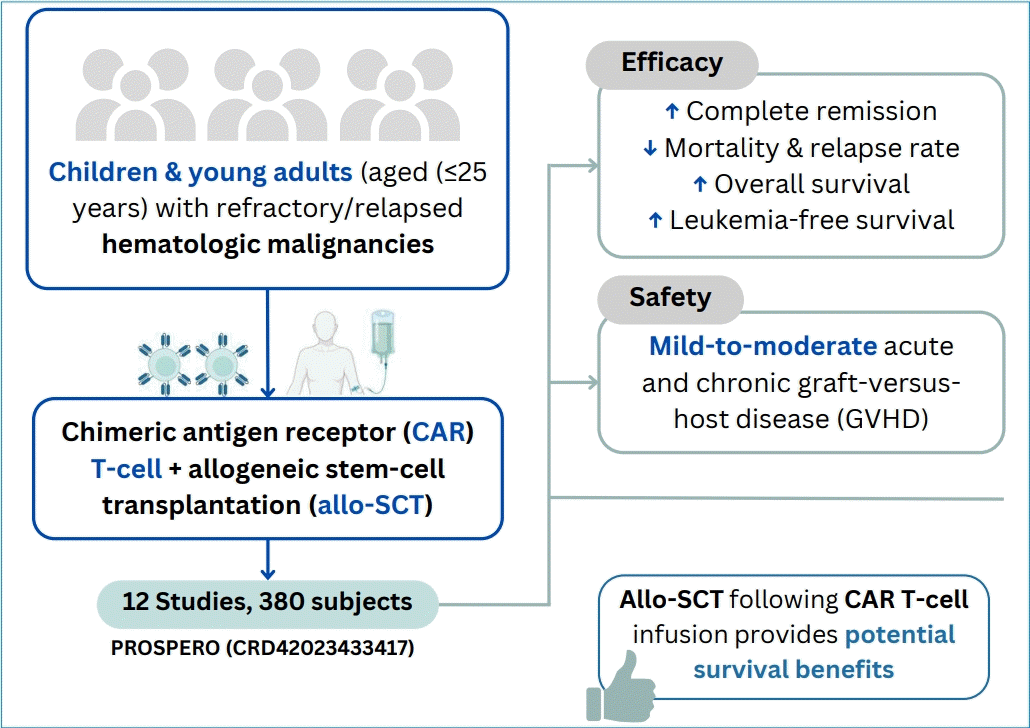
This study aimed to ascertain whether incorporating consolidative allo-SCT after CAR T-cell therapy can augment the therapeutic outcomes of child and young adult patients with relapsed/refractory hematologic malignancy.
Methods
A comprehensive literature search of PubMed, ScienceDirect, Cochrane Library, EBSCOHost, ProQuest, and the grey literature repositories was performed for articles published between May 5, 2014, and May 5, 2024. We included studies reporting consolidative allo-SCT following CAR T-cell therapy for treating hematologic malignancies in subjects aged ≤25 years old. The outcomes of interest were complete remission, survival, relapse, and mortality rates. The estimates were pooled using random-effects meta-analysis. The risk of bias was evaluated using the Newcastle-Ottawa Scale, while the certainty of evidence was assessed using GRADE. This study follows the PRISMA 2020 criteria and is registered in the PROSPERO database (CRD42023433417).
Results
Twelve cohort studies involving 380 patients, primarily those with B-cell acute lymphoblastic leukemia (B-ALL), were included. The CAR T-cell+SCT group showed a trend toward higher complete remission (odds ratio [OR], 2.74; 95% confidence interval [CI], 0.88–8.54; P= 0.08; I2=57%; evidence, very low); lower mortality (OR, 0.58; 95% CI, 0.27–1.27; P=0.17; I2=0%; evidence, low), and decreased relapse (OR, 0.18; 95% CI, 0.06–0.56; P=0.003; I2=41%; evidence, low) rates than those who did not proceed to SCT. In addition, both overall survival and leukemia-free survival rates showed a favorable trend toward the CAR T-cell+SCT group, respectively (hazard ratio, 0.44; 95% CI, 0.25–0.77; P=0.005; I2=0%; evidence, low; and hazard ratio, 0.29; 95% CI, 0.17–0.49; P<0.00001; I2=0%; evidence, low). Common posttransplant toxicities include mild to moderate acute and chronic graft-versus-host diseases.
Conclusion
Although the current level of evidence remains low or very low, allo-SCT following CAR T-cell infusion potentially benefits patient survival. Further clinical studies are required to confirm these findings.
Key message
Question: Does consolidative allogeneic stem cell transplantation (allo-SCT) after chimeric antigen receptor (CAR) T-cell therapy improve outcomes of children and young adult patients with relapsed/refractory hematologic malignancies?
Finding: The meta-analysis showed reduced relapse rates and favorable survival trends with allo-SCT despite low evidence quality.
Meaning: Consolidative allo-SCT after CAR T-cell therapy may enhance survival; however, further clinical studies are needed.
Graphical abstract
Introduction
Cancer remains a worldwide health concern, resulting in a significant burden of disability-adjusted life years (DALYs). This burden amounts to 11.5 million DALYs, 82.2% of cases in low-to-middle-income countries [1]. The current conventional therapy has given acceptable outcomes, and improved the survival rate by more than 70% among children, adolescents, and young adults (CAYA) diagnosed with B-cell malignancies. However, 15%–40% of patients suffer from relapse and 2%–3% remain unresponsive to initial treatment. The prognosis of such patients is typically very poor, with a survival rate no higher than 20% [2,3].
Allogeneic stem cell transplantation (allo-SCT) arises as a potential curative treatment option by substituting the diseased bone marrow with healthy stem cells, effectively resetting the patient's hematopoietic system. Yet, among patients who did not attain minimal residual disease (MRD) negativity before undergoing allo-SCT, their 3-year overall survival (OS) and leukemia-free survival (LFS) stood at a mere 23.5% and 20.6%, respectively [4]. Recently, chimeric antigen receptor T-cell (CAR T-cell) therapy has successfully shown that up to 80% of patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) achieved complete remission with negative MRD [5,6]. Although CAR T-cell imposes substantial burdens on healthcare providers, it is still cost-effective, even in low-to-middle-income countries, suggesting its applicability in resource-limited settings [3,7,8]. A significant downside of CAR T-cell therapy likely involves the potential for relapse, with the most alarming type of relapse being lineage switch [2,3].
The reasoning behind integrating CAR T-cell therapy with SCT rests on their complementary modes of action. CAR T-cell therapy provides a potent, focused assault on leukemic cells, while SCT eliminates remaining malignant cells and encourages the establishment of a strong immune system. This potentially harmonious strategy shows significant potential for attaining more profound and long-lasting remissions, and perhaps even cures, in patients with R/R leukemia. Some suggest that using SCT after CAR T-cell therapy can increase survival and lower recurrence risks [5,6,9-16], while others argue the associated risks might overshadow potential benefits [17-21]. Therefore, our study aims to conduct a systematic review of contemporary clinical research and thoroughly assess the potential benefits of allo-SCT administered post-CAR T-cell infusion and their impact on treatment outcomes among pediatric, adolescent, and young adult populations.
Methods
1. Search strategy and eligibility criteria
A comprehensive literature search was conducted across multiple databases, including PubMed, ScienceDirect, Cochrane Library, EBSCOHost, ProQuest, and grey literature repositories such as Google Scholar, OpenGrey, and WorldCat. Articles published from May 5, 2014 to May 5, 2024, were included in the search. Search terms included ("Chimeric Antigen Receptor" OR "CAR T cell") AND ("Hematologic Neoplasm" OR "Hematologic Malignancy" OR "Hematological Malignancy" OR "Blood Cancer" OR "Leukemia" OR "Lymphoma") along with relevant MeSH (medical subject headings) terms if applicable. Additionally, a snowball search method was employed to identify relevant articles by screening citations of published review articles aligned with the research topic and objectives.
The search process was independently conducted by 3 authors (GM, NA, SK), followed by a screening of articles using the predefined keywords. Duplicate articles were removed, and the remaining articles were screened based on title and abstract to identify potentially eligible manuscripts. Full-text articles meeting the eligibility criteria underwent a thorough evaluation for data synthesis. Reasons for excluding studies were recorded, and discrepancies were resolved through consensus facilitated by a third party (EST or RJ). All selection procedures were carried out manually by the authors, and the outlined process is summarized in Fig. 1.
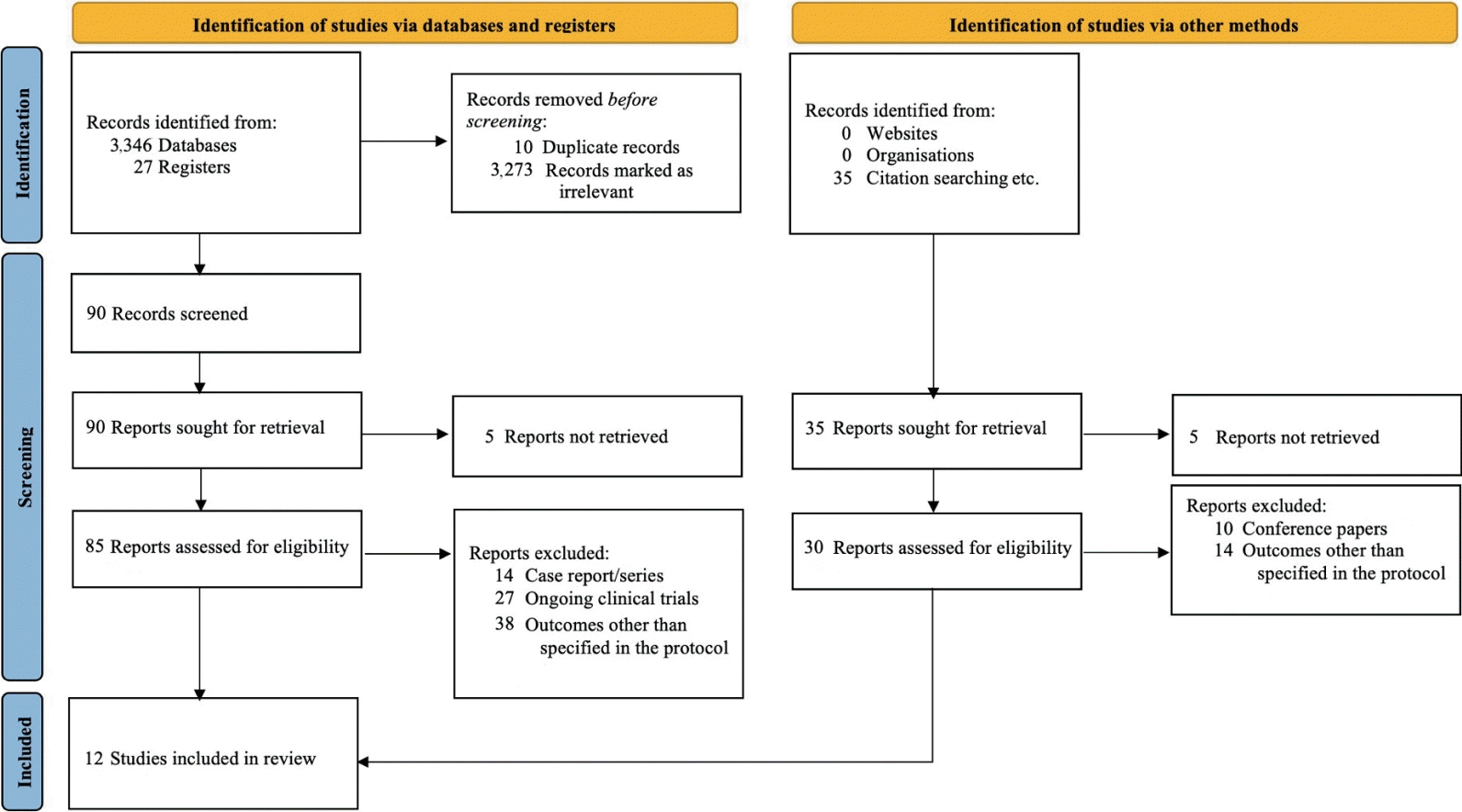
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram of the search strategy and selection process used to identify articles suitable for inclusion in this meta-analysis
The eligibility of studies was determined based on specific criteria, including study type, target population, intervention, comparator, and outcome measures. Both randomized and nonrandomized controlled trials and observational studies were considered suitable for inclusion in further analysis. Studies lacking full-text availability were excluded from this review, along with reviews, commentary articles, and book sections. The study population comprised individuals aged 25 years and below diagnosed with hematologic malignancies, encompassing pediatric and young adult cohorts. No restrictions were imposed based on race, socioeconomic status, religion, geographic location, or underlying health conditions. All trials reporting consolidative allo-SCT following CAR T-cell therapy were incorporated in this review to present a comprehensive overview of its current application in treating hematologic malignancies in CAYA. As a comparison, we included subjects who received CAR T-cell but did not proceed to allo-SCT. The outcomes of interest were complete remission, survival, relapse and mortality rate. Where available, posttransplant adverse events were also extracted. This study synthesized data from electronic databases. Hence the institutional review board was not compulsory.
This review was reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) 2020 criteria [22]. The protocol had been recorded in the PROSPERO (International Prospective Register of Systematic Reviews) (CRD42023433417).
2. Data extraction and quality assessment
Three reviewers (GM, NA, SK) independently extracted data from the articles selected during the screening phase. In cases of disagreement, consensus was reached through discussion facilitated by EST or RJ. The extracted data included: (1) first author and publication year; (2) geographical region of the study; (3) study design; (4) a specific type of hematologic malignancy investigated; (5) sample size; (6) age of participants; (7) Eastern Cooperative Oncology Group performance status; (8) details of the intervention (including target and construction, dosing, and regimen) and any applicable control group; (9) level of blasts prior to CAR T-cell infusion; (10) duration between infusion and SCT; (11) rationale(s) for undergoing SCT; and (12) clinical outcomes following CAR T-cell infusion followed by SCT. All data extraction procedures were conducted manually by the reviewers. Any missing data required for the analysis will be retrieved by contacting the corresponding author of the article.
Newcastle-Ottawa Scale (NOS) was used to determine the quality of observational studies [23]. NOS evaluates the nonrandomized research’s quality over 3 domains: selection, comparability, and outcome. The selection domain (up to 4 stars) does provide some merit to the participant's selection and whether exposure is feasible to measure and outcomes are absent at minimum (in cohort studies). The comparability domain (up to 2 stars) evaluates what controls the study implements for confounding factors, both in design and analysis. The outcome domain (up to 3 stars) assess the reliability of assessment and adequacy as well as consistency of follow-up or data collection across groups. Increased total score (7–9 stars) suggests methodologically strong study, while lower scores indicate increased risk of bias and less validity. Four researchers (GM, NA, SK, EST) independently judged the methodological quality of each study manually with any discrepancies resolved through a consensus agreement mediated by RJ.
3. Statistical analysis
We pooled the data in a meta-analysis, when adequate data was available. Odds ratio (OR) was used to estimate the proportion of patients with complete remission, experiencing mortality and/or relapse. We used the Mantel-Haenszel method to combine the study-specific estimates. We also calculated the survival rate of patients treated with CAR T-cell+SCT by generating hazard ratio (HR) using generic inverse variance method. The effect was considered significant if the value was not equal to 1 with P<0.05. The pooled results were generated into the forest plots and sorted based on the study weight in ascending order. Sensitivity analysis was performed to confirm the robustness of this meta-analysis by excluding studies with a high risk of bias into the analysis.
Random-effects model was selected to accommodate the heterogeneity across studies. The heterogeneity was assessed using Cochrane Q Test and Higgins I² statistics (values 0%–40% indicate low; 30%–60%, moderate; and 50%–100%, considerable heterogeneity). Subgroup analysis will be conducted to find the possible cause of heterogeneity. A funnel plot was used to assess publication bias visually. An asymmetric funnel plot indicated the possibility of publication bias [24]. This would be confirmed through the Begg and Mazumdar Rank Correlation Test and Egger’s Test of the Intercept to determine the presence of publication bias statistically [25,26].
All statistical tests were done using Review Manager (RevMan) ver. 5.4.27) When meta-analysis could not be conducted, a narrative synthesis evaluating clinical outcomes reported in the included studies will be performed, instead. The findings of the included studies will be presented in a table, and the studies listed will be sorted based on the alphabetical order of the first author.
4. Certainty of evidence
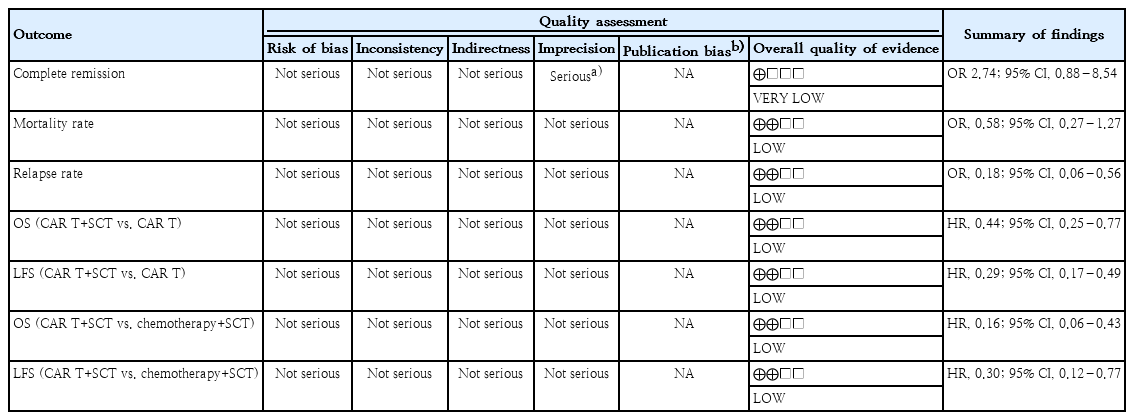
Grading of recommendations, assessment, development, and evaluations (GRADE) was used to evaluate the confidence in cumulative evidence. Judgment was made considering the presence of study limitations, consistency, directness, imprecision, and/or reporting bias. Overall certainty of evidence was shown as high, moderate, low, or very low quality [28].
Evidence is initially evaluated based on the type of study conducted: randomized controlled trials are classified as high certainty evidence, while observational studies are classified as low certainty. In the GRADE framework, evidence can be downgraded due to several concerns: risk of bias due to methodological issues in more than half of the included studies; inconsistency indicated by significant unexplained variability in results across studies; indirectness when the evidence does not precisely align with the research question; imprecision reflected by wide confidence intervals that reduce confidence in the effect estimate; and publication bias, suspected when relevant studies are missing or results appear unrealistically positive.
Results
A total of 3,408 records were identified from electronic databases. After removing duplicates and irrelevant articles, 115 records remained for screening and further assessed for eligibility. Ultimately, 12 studies which met the inclusion criteria were included in the review [9-20]. The search flowchart and study selection are illustrated in Fig. 1.
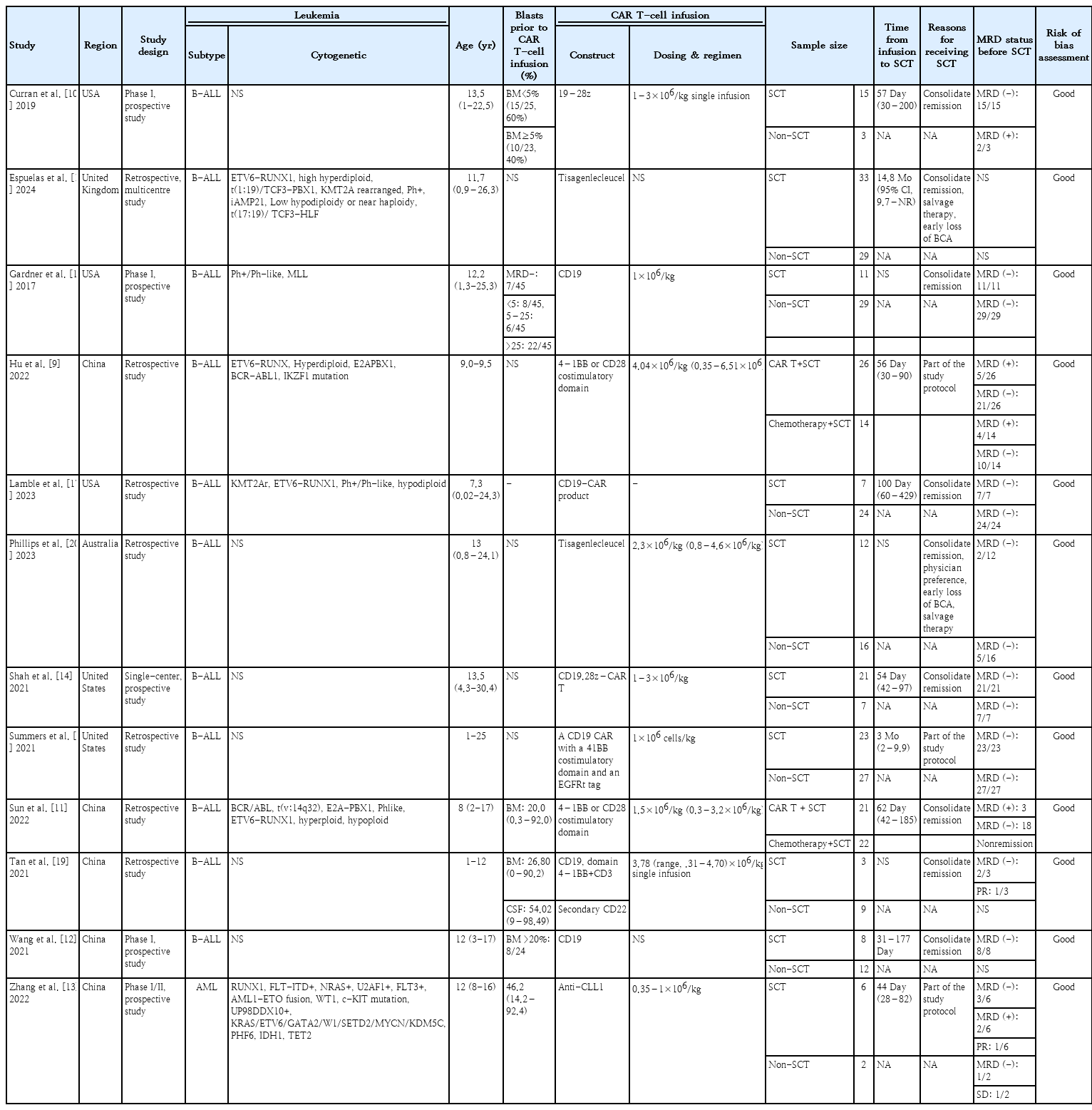
Twelve cohort studies involving 380 subjects were conducted across diverse regions, spanning America, Asia, and Europe. All studies enrolled participants aged 25 years or younger. Except for one study [13], all reported cases of R/R B-ALL, with or without extramedullary involvement, notably in the central nervous system. The blast cells' proportion before CAR T-cell infusion varied from 0.03% to 99.00%. Across all studies, CAR T-cell therapy targeted the CD19 epitope, with dosages ranging from 0.3 to 9.9×106/kg in single infusions. The majority of patients responded well to the therapy and the decision of receiving SCT after CAR T-cell therapy were influenced by either disease relapse or physicians’ preference to consolidate remission. Neutrophil and platelet engraftment were achieved typically in 11–21 days and 8–89 days, respectively. All of the included studies showed good quality. Table 1 provides detailed characteristics of the included studies.
We observed a trend of higher complete remission (OR, 2.74; 95% CI, 0.88–8.54; P=0.08; I2=57%) (Fig. 2A) and lower mortality rate (OR, 0.58; 95% CI, 0.27–1.27; P=0.17; I2=0%) (Fig. 2B) in the CAR T-cell+SCT group compared to those who did not proceed to SCT, although the difference was not statistically significant. There was a significant reduction of relapse rate among patients who received SCT after CAR T-cell therapy (OR, 0.18; 95% CI, 0.06–0.56; P=0.003; I2=41%; Fig. 2C). In addition, both OS and LFS showed favorable trend towards the CAR T-cell+SCT group, respectively (HR, 0.44; 95% CI, 0.25–0.77; P=0.005; I2=0% and HR, 0.29; 95% CI, 0.17–0.49; P<0.00001; I2=0%) (Fig. 2D and E). Our meta-analysis also showed favorable outcomes of CAR T-cell therapy as a bridge before receiving consolidative SCT. Compared to the patients only receiving chemotherapy before SCT, those bridged with CAR T-cell therapy showed improvement of OS and LFS, respectively (HR, 0.16; 95% CI, 0.06–0.43; P=0.0003; I2=0% and HR, 0.30; 95% CI, 0.12–0.77; P=0.01; I2=0%) (Fig. 3).
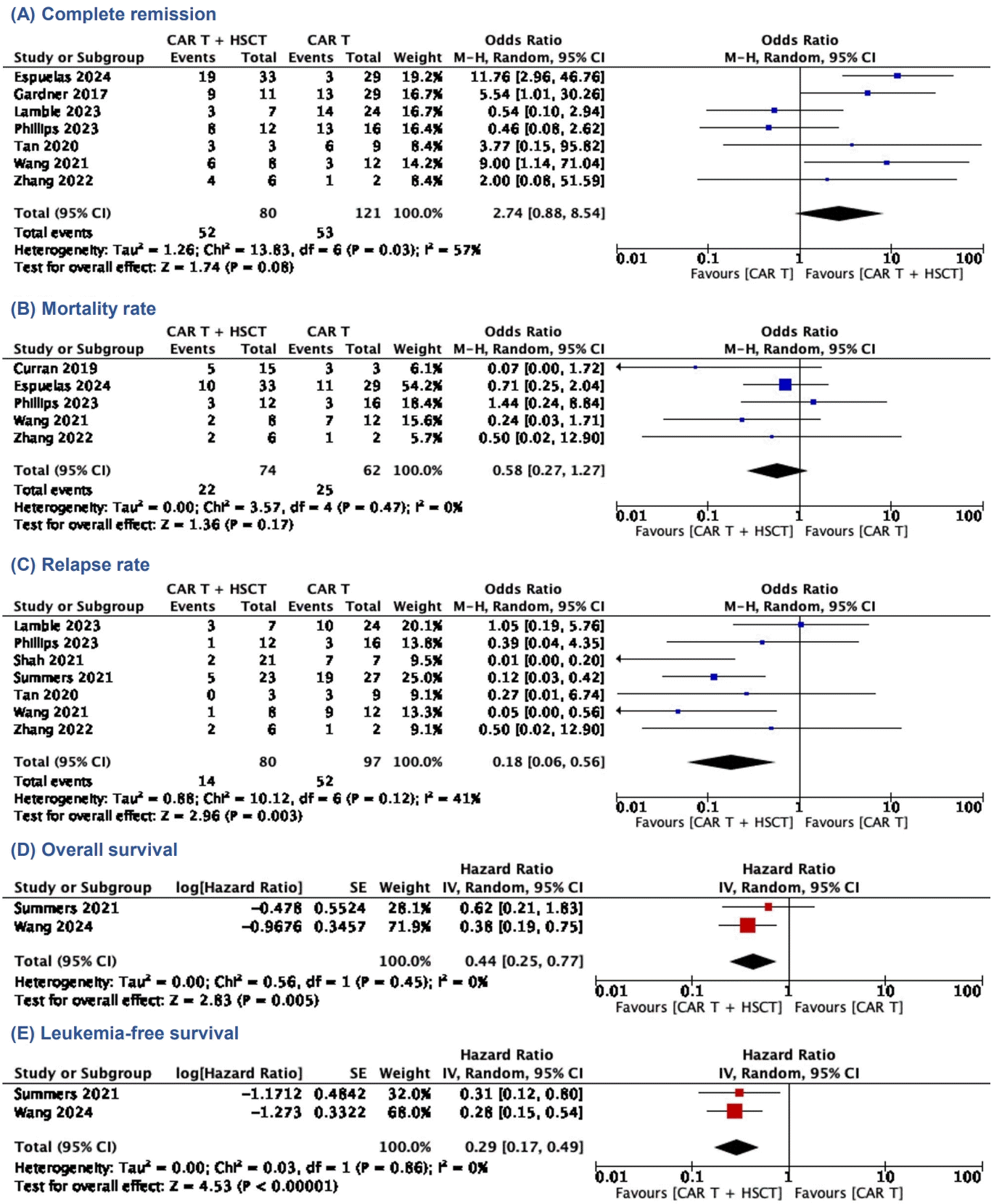
Clinical efficacy of SCT following CAR T-cell infusion. (A) Complete remission. (B) Mortality rate. (C) relapse rate. (D) Overall survival. (E) Leukemia-free survival. CAR, chimeric antigen receptor; SCT, stem-cell transplantation; M-H, Mantel- Haenszel; CI, confidence interval; df, degrees of freedom.
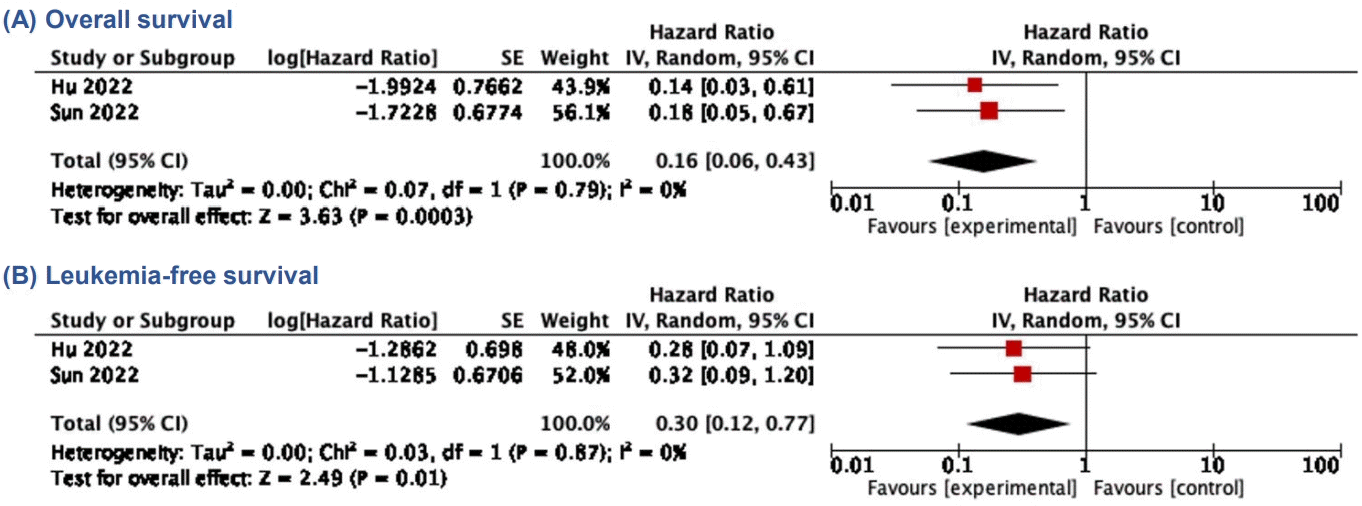
CAR T cell versus chemotherapy as bridging therapy before SCT. (A) Overall survival. (B) Leukemia-free survival. CAR, chimeric antigen receptor; SCT, stem-cell transplantation; SE, standard error; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
In the CAR T-cell group, common posttransplant toxicities included manageable rates of acute and chronic graft-versus-host disease (GVHD). Hu et al reported the incidence of grade II–III and III–IV acute GVHD were 26% and 7%, respectively. In a long-term follow-up, severe chronic GVHD accounted for 11% (3 out of 26 subjects). Sun et al. [11] observed similar findings whereas the incidence of grade II–IV acute GVHD was 28.6%, while no patient experienced extensive chronic GVHD in 2 years. Serious adverse events were rare, but included thrombotic microangiopathy and infectious complications.
This meta-analysis included cohort studies, which initially provided low quality evidence. All except one study showed no concern in regards to imprecision. We detected no substantial risk of bias, heterogeneity, or indirectness among studies. Publication bias could not be evaluated as the number of included studies was less than 10. The evidence generated from this meta-analysis was judged to be very low-to-low, as outlined in Table 2.
Discussion
Despite the presence of chemotherapy and allo-SCT, the prognosis for CAYA with R/R B-ALL remains highly unfavorable, especially for those unresponsive to initial treatment. The sustained survival of these individuals hinges on achieving complete remission through salvage chemotherapy followed by allo-SCT. Regrettably, a notable portion of patients never undergo potentially life-saving allo-SCT due to the inability to attain a second complete remission after salvage chemotherapy. Additionally, for patients who do undergo allo-SCT, those identified with MRD positivity confront a significantly poorer prognosis compared to individuals with no signs of MRD at the time of allo-SCT [29]. This emphasizes the critical need for innovative therapeutic strategies tailored to this patient demographic [30].
The observed trend of higher complete remission rates and lower mortality in the CAR T-cell+SCT group underscores the potential synergistic effects of combining these 2 innovative therapies [31]. The significant reduction in relapse rate among patients who received SCT after CAR T-cell therapy further reinforces the value of this sequential approach, as maintaining long-term disease control is crucial for improving patient prognosis [32]. Additionally, the favorable trends observed in OS and LFS for the CAR T-cell+SCT group suggest that this combination may enhance the durability of clinical responses, potentially leading to improved long-term outcomes [33]. The meta-analysis also highlights the potential benefits of using CAR T-cell therapy as a bridge to consolidative SCT. The safety profile of the CAR T-cell+SCT treatment was generally well-tolerated, as indicated in literature [29,34].
At the molecular level, CAR T-cells can initiate a rapid and highly specific cytotoxic response. Upon antigen engagement, these cells release perforin and granzymes to induce apoptosis in malignant targets, while also producing inflammatory cytokines, such as interferon-gamma and tumor necrosis factor-alpha that amplify anti-leukemic immunity [35,36]. This targeted immune response can lead to deep remissions and MRD negativity, effectively reducing tumor burden and creating an optimal immunologic window for allo-SCT. However, CAR T-cell efficacy can be limited by antigen escape, T-cell exhaustion, and limited persistence [6,14]. Allo-SCT addresses these gaps by reconstituting the immune system with donor-derived cells capable of exerting a broad graft-versus-leukemia effect. Unlike CAR T-cells, this response is not restricted to a single antigen and involves recognition of minor histocompatibility antigens and tumor-associated peptides by donor T-cells and natural killer cells [37,38]. This diverse and sustained immune surveillance is particularly effective when performed after CAR T-cell-induced cytoreduction, as it targets any residual leukemic cells that may have evaded initial therapy [39,40].
Together, this sequence leverages the potent, short-term cytotoxicity of CAR T-cells and the durable, systemic immunologic control of allo-SCT to achieve superior and sustained remission outcomes. This strategy also positions patients for better outcomes by mitigating the risks associated with bridge therapies like conventional chemotherapy, which have shown limitations in producing sustained responses in prior studies [9,11,41]. Not only addresses the critical need for effective pre-SCT therapies, but it also emphasizes the growing preference for immunotherapeutic strategies that minimize the associated toxicities of traditional treatments, ultimately improving the overall therapeutic landscape in oncology. Moreover, findings emphasize the necessity of optimizing the cell product quality in adoptive T-cell therapies, as this can further reduce adverse effects and clinical costs, making these therapies more accessible and practical for a broader range of patients. Furthermore, the integration of CAR T-cell therapy within treatment regimens is indicative of a significant shift towards personalized medicine in oncology, reflecting the need to develop individualized approaches that harness the patient's immune system to effectively combat malignancies, which is particularly crucial for improving outcomes in solid tumors [42].
Our study differs from existing literature by offering a targeted analysis of the synergistic potential of consolidative allo-SCT following CAR T-cell therapy. Additionally, we address gaps in prior research by systematically examining relapse rate, survival endpoints (OS and LFS), and treatment-related toxicities in post-CAR T SCT settings—an area still underrepresented in current literature, specifically in the CAYA population.
This meta-analysis has several limitations. First, the relatively small number of patients treated with CAR T-cell followed by allo-SCT limits the statistical power and generalizability of the results. It may reduce confidence in the observed treatment effects, increase susceptibility to bias, and restrict subgroup analyses. Second, available data reporting the safety profile was still limited. It is not clear whether the complications occurred following allo-SCT were purely related to the transplantation process itself or influenced by the previous treatments received and/or the comorbidities of the subjects. Finally, the studies were mainly conducted in developed regions, such as China, United States, and European countries which made its availability limited in low-to-middle-income countries.
In this study, we illustrate that employing a sequential approach involving CAR T-cell therapy followed by consolidative allo-SCT offers potential benefits as effective disease management in CAYA diagnosed with R/R B-ALL. Despite the constraints inherent in our study, additional clinical trials are warranted to ascertain the advantages of CAR T-cell therapy as a bridging intervention preceding allo-SCT fully.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: GM, NA, SK; Methodology: GM, NA, SK, EST, RJ; Literature Search: GM, NA, SK; Data Analysis: GM, NA, SK, EST, RJ; Writing - original draft preparation: GM, NA, SK; Writing - review and editing: GM, NA, SK, EST, RJ; Validation: GM, NA, SK, EST, RJ.