Effect of vitamin C supplement in treatment of childhood pneumonia requiring hospitalization: a randomized controlled trial
Article information
Abstract
Background
The role of vitamin C in children with community-acquired pneumonia (CAP) in children is controversial; moreover, a standard dose is lacking.
Purpose
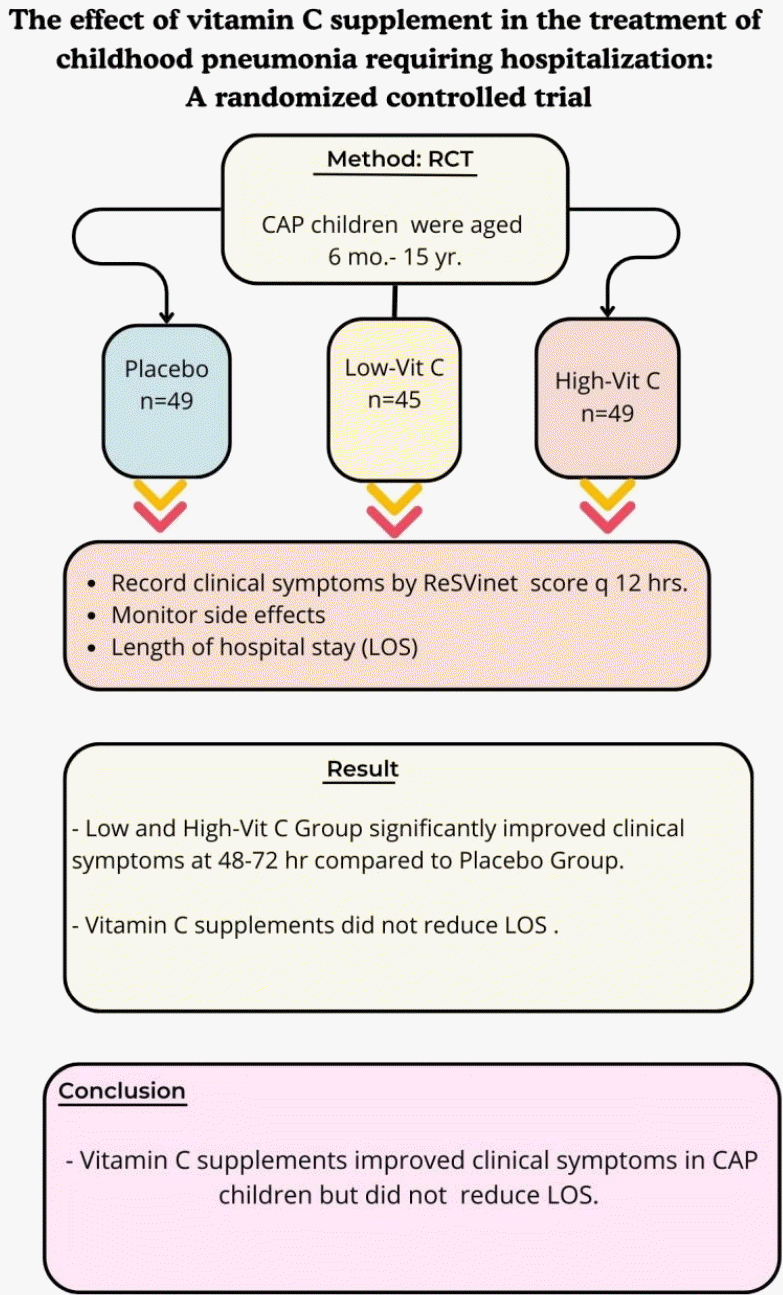
This study aimed to evaluate the ability of vitamin C to reduce symptom severity and length of hospital stay among children with CAP as well as determine its optimal dose.
Methods
This randomized controlled clinical trial was conducted between July 2020 and October 2023. The participating patients were aged 6 months to 15 years, had CAP, and required hospitalization at Naresuan University Hospital. The patients were randomly assigned to placebo, low-dose vitamin C (15 mg/kg/dose every 6 hours), and high-dose vitamin C (30 mg/kg/dose every 6 hours) groups. Treatment was provided until discharge and doses maximized after 3 days. The patients' clinical symptoms and side effects were recorded every 12 hours.
Results
This study included 143 patients (median age, 3 years). The clinical severity score improved significantly in the low- and high-dose vitamin C versus placebo groups at 48–72 hours. Vitamin C supplementation did not reduce the length of hospital stay in any group.
Conclusion
Vitamin C supplementation did not reduce the length of hospital stay among patients with CAP who required hospitalization. However, it improved the mean clinical severity score, with the greatest reduction observed at 48-hour posttreatment. A dose of 15 mg/kg was demonstrated effective with minimal side effects.
Key message
This study assessed the effects of vitamin C on children with community-acquired pneumonia (CAP). Vitamin C supplementation improved clinical symptoms within 48–72 hours compared to placebo but did not reduce the length of hospital stay (LOS). These findings suggest that vitamin C is beneficial for managing CAP severity, but does not affect LOS.
Graphical abstract. RCT, randomized controlled trial; CAP, community- acquired pneumonia; Low-Vit C, low-dose vitamin C; High-Vit C, high-dose vitamin C.
Introduction
Pneumonia is one of the most common lower respiratory tract infections which is caused by a variety of pathogens such as viruses, bacteria and fungus. This disease causes a high burden of hospitalization and mortality in younger children. In 2019, World Health Organization stated that pneumonia was the cause of death in children under 5 years with around 740,000 deaths per year worldwide [1]. In Thailand, the Department of Disease Control reported the incidence of pneumonia at around 400,000–500,000 people per year, with elderly patients over 65 years old and children under 5 having high morbidity and mortality [2].
Vitamin C is a water-soluble vitamin that has an antioxidant function, preventing tissue damage from oxidative stress and limiting the production of new free radicals. It also affects the immune system by stimulating neutrophil migration to the infection site and regulating macrophage function [3,4]. Vitamin C has a beneficial effect on respiratory tract infection due to its antioxidant properties. In viral or bacterial infection, the activation of neutrophils causes the consumption of extracellular vitamin C which reduces the vitamin C level [5]. These suggest that providing vitamin C supplements may alleviate the symptoms of respiratory tract infection.
Guidelines for treatment in community-acquired pneumonia (CAP) suggest treating pneumonia with appropriate antibiotics, oxygen therapy and supportive care [6,7]. There was much research on the treatment of pneumonia to improve the severity and clinical outcome [8-13]. The study of Mahalanabis et al. [9] with children aged 2 to 35 months did not show any benefit of vitamin C and vitamin E in severe acute lower respiratory tract infections, in contrast to other studies on childhood pneumonia that did show the benefit of vitamin C [11-13]. For instance, a study by Hotiyana et al. [14] in children under 5 years of age, using 25 mg of oral vitamin C, reported a reduction in hospital stay duration. Similarly, other studies using different oral vitamin C dosages have also shown benefits in reducing the length of hospital stay (LOS) [13]. However, most prior studies have not examined the correlation between vitamin C levels and hospital duration. Furthermore, most studies in children have used single dose oral vitamin C and primarily focused on children under 5 years old.
The route of vitamin C administration significantly influences plasma concentration levels. Intravenous vitamin C produces higher plasma concentrations than oral supplementation, which is limited by gastric absorption [15]. In critically ill patients, and reduced oral intake further hinder effective vitamin C absorption. To address these limitations, we opted for intravenous vitamin C administration in this study. Additionally, pneumonia etiology varies by age group: in younger children (2 months to 5 years), viral pathogens are more common, while in older children (over 5 years), bacterial and atypical pathogens predominate. Viral pneumonia is typically managed with supportive care, while bacterial pneumonia often requires a more specific treatment and may result in more complications [7]. Pneumonia surveillance studies in Thailand indicate that the highest incidence occurs in children under 5 years old, followed by children aged 5–9 years 10–14 years [16,17].
Given these variations in pneumonia etiology, age-related differences, and vitamin C administration routes, the role of vitamin C in the treatment of pneumonia remains controversial. Further evidence is needed to determine its efficacy and optimal dosage in pediatric patients. This study aimed to assess the effects of vitamin C in childhood pneumonia, including both younger and older hospitalized children, by evaluating its impact on symptom scores, the LOS, and the optimal dosage of vitamin C supplementation for treatment.
Methods
1. Study design and setting
This study was a randomized controlled clinical trial in Naresuan University Hospital, a tertiary care hospital in Thailand, conducted between July 2020 to October 2023.
2. Statement of ethics
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by Institutional Review Board of Naresuan University (approval number: P3-0149/2563). The study's clinical trial registration number is TCTR20210427006 registered with Effect of Vitamin C Supplementary in Childhood Pneumonia. Participant registration took place from Jul-2020 to Oct-2023. All parents, guardians or next of kin provided written informed consent for the minors to participate in this study.
3. Definition
CAP: the presence of signs and symptoms of pneumonia in a previously healthy child due to an infection which has been acquired outside a hospital in the wider community [18].
4. Participants
This study enrolled children aged 6 months to 15 years hospitalized with mild to moderate CAP who required hospitalization. Children younger than 6 months were excluded due to concerns about immature renal function and potential vitamin C-related side effects in this age group. Given the limited data on vitamin C use in older children (over 5 years) with pneumonia, this study specifically included children up to 15 years of age. Although this study was conducted during the pandemic period, it is important to note that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection increases the risk of acute respiratory complications compared to other infections and is associated with numerous sequelae, such as chronic respiratory failure [19,20]. This infection necessitates specialized nursing, medical care, and the use of protective equipment during the pandemic. Consequently, we excluded patients with SARS-CoV-2 infection from this study.
Additional exclusion criteria were patients with (1) malnutrition or severe protein energy malnutrition (2) patients that have risk factors for severe pneumonia such as chronic lung disease, congenital malformation of respiratory tract disease, congenital heart disease with hemodynamic significant, malignancy, chronic liver disease, cerebral palsy, uncontrolled epilepsy, immunodeficiency, or were receiving an immunosuppressive drug (3) requiring intubation (4) had complications such as parapneumonic effusion or pneumothorax (5) were hemodynamic unstable, needing inotropic drug (6) had renal insufficiency (7) a history of vitamin C allergy (8) had previously received vitamin C in the week before admission, (9) had an underlying disease of G6PD deficiency and vitamin B12 deficiency, or (10) currently had SARS-CoV-2 infection, (11) severe severity of CAP (ReSVinet score more than 14 points).
5. Intervention and data collection
Each patient enrolled in the study was stratified by age group: 6 months to 5 years, >5 to 10 years, and >10 to 15 years. They were then randomly assigned to 1 of 3 treatment groups on a 1:1:1 basis. For identification purposes, these treatment groups were labeled as the placebo group (receiving normal saline), the low-vit C group (receiving vitamin C 15 mg/kg/dose every 6 hours), and the high-vit C group (receiving vitamin C 30 mg/kg/dose every 6 hours). The high-dose vitamin C regimen was based on a study by Wald et al. [21] on pediatric septic shock, where 30 mg/kg/dose every 6 hours, combined with thiamine and hydrocortisone, reduced mortality. Additionally, studies on critically ill adults have used vitamin C doses ranging from 3 to 10 g/day, approximately equivalent to 15–50 mg/kg/dose every 6 hours. Based on these findings, we defined 15 mg/kg/dose every 6 hours as the low-vit C group and 30 mg/kg/dose every 6 hours as the high-vit C group.
The treatment period was until discharge or maximum received 3 days. All patients were initially screened for SARS-CoV-2 infection. Blood samples were collected and tested for vitamin C levels, blood urea nitrogen and creatinine before starting the intervention and after the last dose of treatment. Patient information was collected, including gender and age, nutrition status, underlying diseases, length of current hospital stay, laboratory data, oxygen therapy and treatment. Clinical symptoms were recorded by using the ReSVinet Scale22) for evaluation of clinical severity at time of enrollment and every 12 hours. The evaluation process utilized 7 parameters (feeding intolerance, medical intervention, respiratory difficulty, respiratory frequency, apnea, general condition, fever) that were assigned different values (from 0 to 3) for a total of 20 points, if score more than 14 points defined as severe severity. All groups received standard care for CAP and supportive care (oxygen supplementation, intravenous fluid and monitoring). Side effects were monitored and clinical management of any side effects was noted.
6. Outcome measures
The primary measurable outcomes of this study were (1) the identifiable benefits of vitamin C supplementation in the treatment of CAP, and (2) the optimal dose of vitamin C supplementation in that treatment. Secondary outcomes included vitamin C supplementation associated with clinical severity.
7. Endpoints
The primary endpoint of this study was the LOS, defined as the time from enrollment in the study until discharge from the hospital. The secondary endpoint was the reduction of symptoms or clinical improvement.
8. Sample size
The sample size was calculated prior to data collection using analysis of variance (ANOVA) in the G*Power program (ver. 3.1.9.7). With 3 groups, an effect size of 0.284, and an alpha of 0.05, the power analysis indicated a total sample size of 123 (41 per group) to achieve 80.23% power. Accounting for a 10% dropout rate, the minimum sample size was increased to 45 participants per group.
A type I error rate (α) of 0.05 was selected for this study, which is a commonly accepted threshold in scientific and biomedical research. This means we accepted a 5% probability of committing a false positive, striking a balance between minimizing false positives and detecting meaningful effects. A type II error rate (β) of 0.2 (or 80% power) was chosen, ensuring an 80% probability of detecting a true effect when one exists. This power level is a standard in research, providing a good balance between detecting true effects and maintaining reasonable sample size.
Prior to data collection, a power analysis was conducted to estimate the minimum required sample size while controlling for both types I and II errors. While increasing power reduces the risk of missing true effects, it requires larger sample sizes or stronger effects. Therefore, setting a moderate power of 80% ensured confidence in the findings without necessitating an excessively large sample size.
9. Randomization
1) Sequence generation and implementation
A randomization list will be generated using a computer program (StatsDirect) by a staff member who is not involved in the clinical aspects of the trial. The allocation ratio will be 1:1:1.
2) Allocation concealment mechanism
To maintain concealment of the allocation sequence, participants will be enrolled and assessed in a sequential manner. White, opaque, sealed, and stapled envelopes will be used, and these will only be opened after obtaining informed consent from parents or caregivers and recording the basic demographic information on the case report form. Participants will be assigned consecutive randomization numbers upon enrollment. The study products will be labeled as product A, product B, or product C, and participants will receive them according to the randomization list.
3) Blinding
The study products (product A being a placebo, product B a low dose of vitamin C, and product C a high dose of vitamin C) will be packaged in identical bottles, with contents that appear the same. Researchers, caregivers, outcome assessors, and the staff member handling statistical analysis will remain blinded to the intervention until the study is completed. The details of intervention assignments will be stored in a sealed envelope in a secure location within the administrative area of the department. Personal information of potential and enrolled participants will be kept in a locked facility at the study site, accessible only to the researchers involved.
4) Compliance
Participant adherence to the study protocol was monitored through the analysis of medical records. A trained nurse on the research team administered the placebo and vitamin C, verifying dosages and recording administration times prior to dispensing the medication. In accordance with previous studies, participants were considered compliant if they received the prescribed intervention as intended.
10. Statistical analysis
Prior to data analysis, datasets were examined for outliers and missing values using descriptive statistics and box plots. errors resulted from typos, they were corrected; otherwise, the affected data points were excluded from the analysis.
Statistical analysis was performed using the R ver. 2023. 09.0+463 (R Foundation for Statistical Computing, Austria). ANOVA was performed, and residuals were examined for normality using the Shapiro-Wilk and Kolmogorov-Smirnov tests. When the residuals did not follow a normal distribution, the nonparametric method was utilized. The results were expressed as median (interquartile range) for continuous variables and the frequency (%) for categorical variables. Differences between groups were analyzed using Kruskal-Wallis statistics for quantitative measurement whereas the chi-square or exact tests were used for qualitative measurement. Association between pairs of variables was carried out using Spearman’s correlation. Statistical significance was indicated at a P<0.05.
For this study, the Kruskal-Wallis test was used to compare the medians across 3 groups, as the data did not meet the assumption of normality. However, while this test is robust to nonnormal distributions, the false-positive rate may be inflated with multiple comparisons, requiring careful interpretation of results. Additionally, Bonferroni correction was applied for mean comparisons, while nonparametric methods were used to compare difference in medians.
Results
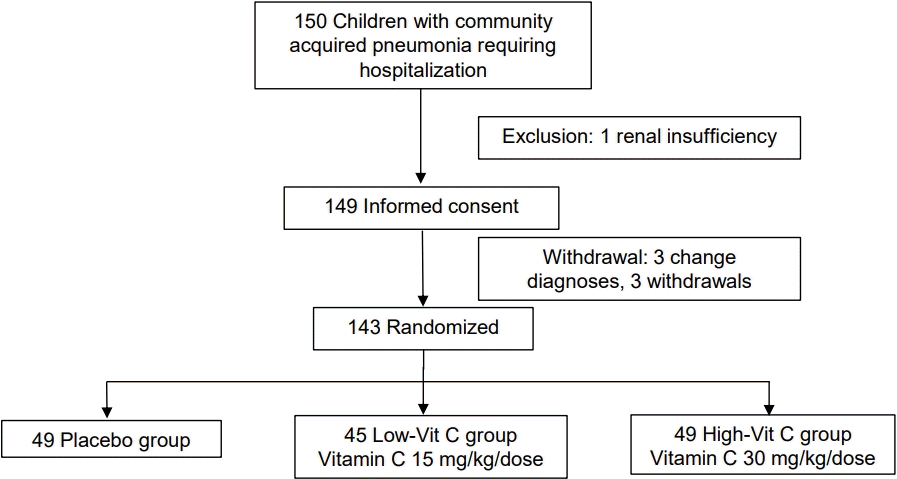
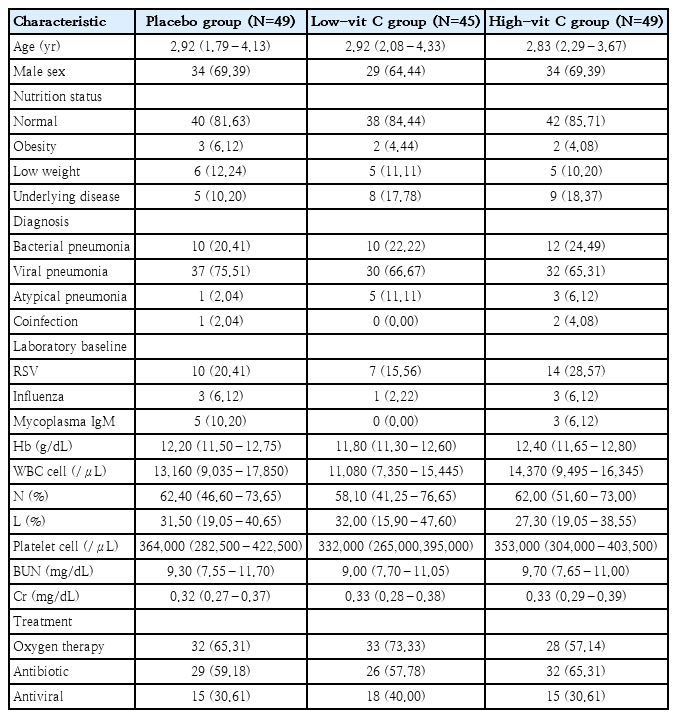
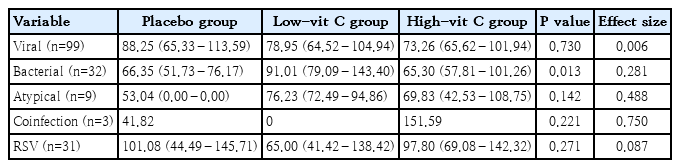
A total of 150 patients were recruited, 1 patient was excluded due to renal insufficiency, and 6 patients were withdrawn (3 voluntarily withdrew and 3 were diagnosed with a different pulmonary disease). Consequently, 143 patients were included in the final analysis and were divided into 3 groups: 49 in the placebo group, 45 in the low-vit C group, and 49 in the high-vit C group (Fig. 1). The median age of patients in the placebo group was 2.92 years, in the low-vit C group it was also 2.92 years, and in the high-vit C group was 2.83 years. There were 97 male patients and 46 female patients, and 120 participants had normal nutrition status. Of the 99 patients diagnosed with viral pneumonia, 37 were in the placebo group, 30 in the low-vit C group and 32 in the high-vit C group. Also, 32 patients were diagnosed with bacterial pneumonia and these were distributed as 10 in the placebo group, 10 in the low-vit C group and 12 in the high-vit C group. In screening for other viral infections, respiratory syncytial virus (RSV) was found in 20.41% of the patients in the placebo group, 15.56 % in the low-vit C group and 28.57 % in the high-vit C group at enrollment. Oxygen therapy; mainly oxygen corrugate, was needed for more than 50% of the patients in each group. Other baseline characteristics were the same for all 3 groups, except for white blood counts which was found to be lower in the low-vit C group than the other groups (Table 1).
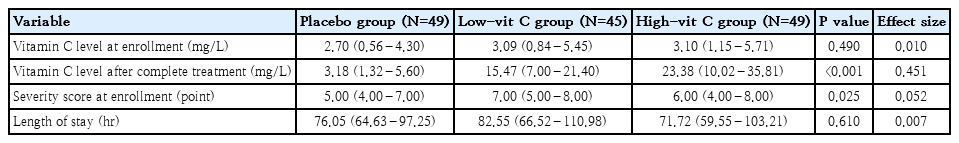
At enrollment, the clinical severity score of the low-vit C group was higher than that of the other 2 groups. The difference was statistically significant (P=0.025). The clinical score values of each group were recorded every 12 hours. Therefore, we use a comparison between the mean clinical score at the time of evaluation and the score at the time of enrollment (mean difference score). The mean difference changes of clinical score from the enrollment are shown in Fig. 2. Both the low-vit C and high-vit C groups showed improvement in their clinical scores more rapidly than seen in the placebo group over the 48 hours to 72 hours of the treatment period. The greatest change in clinical score occurred at 48 hours of treatment, in the low-vit C group (P=0.039). That change was greater than the change recorded for the high-vit C group, and both of these groups showed a greater improvement in clinical score than was recorded in the placebo group. LOS was lowest in the high-vit C group (71.72 hours), the placebo group (76.05 hours) and the low-vit C group (82.55 hours). None of these values showed a statistically significant difference. (Table 2).
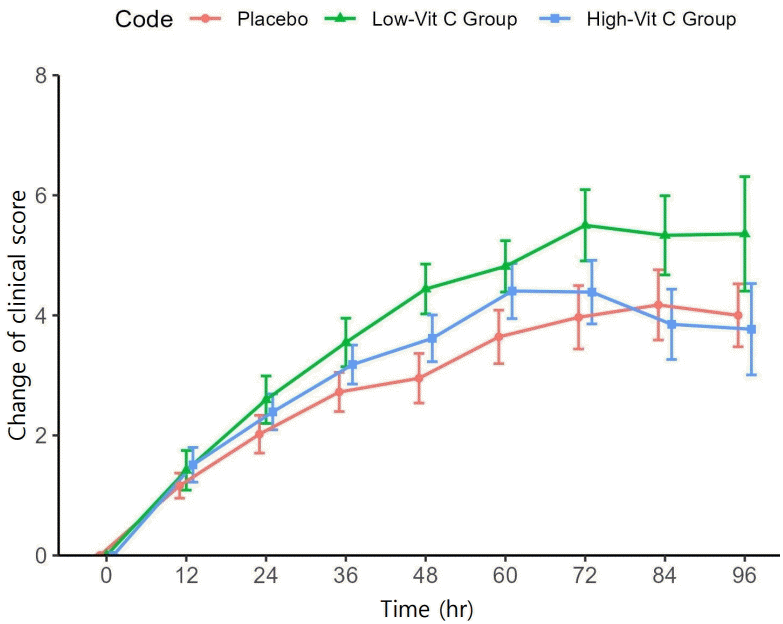
Changes in clinical score by group compared with baseline (time 0). Low-vit C, low-dose vitamin C; High-vit C, high-dose vitamin C.
Recordings at the time of enrollment showed a weak correlation between the vitamin C levels and clinical severity scores of the patients (Spearman correlation, 0.029; P=0.730). Subsequently, after the intervention period, a converse correlation was shown between the vitamin C levels of the patients and their clinical severity scores (Spearman correlation, -0.067; P=0.437).
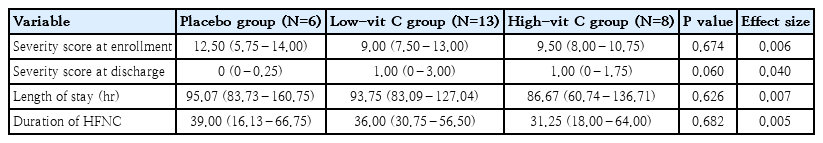
Subgroup analysis of the patients who required a high-flow nasal cannula indicated that these patients had a higher clinical severity score than all other patients in all groups. The median length of stay in the high-vit C group was 86.67 hours and 93.75 hours in the low-vit C group. Both times were shorter than the placebo group (95.07 hours). The values and differences were not statistically significant. Also, the duration of the need for a high-flow nasal cannula was shorter in both the high-vit C group and low-vit C group than in the placebo group, with no statistical significance demonstrated by those values (Table 3).
In each subgroup of diagnoses of viral pneumonia and RSV pneumonia, both the high-vit C group and low-vit C group demonstrated a reduced length of stay, shorter than the placebo group. However, again, there was no statistical significance in those differences (P=0.730). For those patients diagnosed with bacterial pneumonia, the length of stay of the high-vit C group was 65.30 hours, for the low-vit C group it was 91.01 hours and for the placebo group (66.35 hours). These values indicate that the low vitamin C supplement did not help the length of stay in bacterial pneumonia. (Table 4).
Complications and side effects such as gastrointestinal symptoms and renal abnormalities were monitored. Urine output and renal function showed normal levels after the intervention. Some side effects were found in both the high-vit C group and the low-vit C group. Two patients developed mild diarrhea, one in each of the high-vit C and low-vit C groups. As well. 2 patients in the high-vit C group had urine calcium oxalate identified 3 days after enrollment. However, no patient withdrew from the study due to any side effects from the intervention. Also, no patient had any drug allergies.
Discussion
This study is the first study of vitamin C supplementation in children older than 5 years whereas previous studies were mainly of children younger than 5 years [9,11,12]. However, in this study found only 18 patients (12.6%) that older than 5 years and the median age of the participating patients in this study was less than 5 years (3 years). This trial reflected the incidence of CAP requiring hospitalization in Thailand occurring mainly in younger children. The majority of infections were viral, consistent with previous studies [23-26].
The baseline vitamin C level of the patients in this study at enrollment showed as normal or marginally below normal. Similarly, the study by Wahed et. al. [12] showed that the patients with CAP in that study had vitamin C levels normal or marginally below normal for members of the community. This is in contrast with the study of Carr et al. [27] that showed significantly lower vitamin C levels in their cohort of patients with pneumonia than would be found in healthy people. This study demonstrated that after vitamin C supplementation, the levels significantly increased (P≤0.001). This study provides clearer evidence that intravenous vitamin C can significantly increase vitamin C levels in the bloodstream.
Previous studies have not reached a consensus regarding the effectiveness of vitamin C in reducing hospital length of stay. Several studies [12-14,27,28] have reported a reduction in hospital stay, while others found no significant effect [6,29,30]. This study corroborates the findings of previous research, indicating that vitamin C supplementation does not reduce the LOS. However, in contrast, it was observed that vitamin C supplementation significantly improved clinical symptoms, with statistical significance. The clinical severity score in both the low-vit C and high-vit C groups improved within 48–72 hours after receiving vitamin C supplementation. However, upon discontinuation of vitamin C, a deterioration in clinical symptoms was observed, particularly in the high-vit C group. This finding suggests that vitamin C plays a beneficial role in the management of CAP.
We hypothesize that the lack of impact on hospital stay is potentially due to insufficient duration of vitamin C administration. Given vitamin C’s half-life of approximately 2 hours, its active concentration in the bloodstream rapidly decreases due to both metabolic breakdown and renal excretion. While traces of vitamin C or its metabolites may be detectable in urine for up to 24 hours, clinically significant levels are eliminated from the bloodstream within approximately 10 hours of discontinuation. Therefore, stopping the administration of vitamin C may result in symptom recurrence and no reduction in hospital stay duration. We recommend further research to investigate the effects of administering vitamin C for a longer duration, specifically 5 to 7 days, to clarify its potential benefits.
Subgroup analysis in our study revealed no significant benefit of vitamin C supplementation in cases of bacterial pneumonia (primarily caused by Streptococcus pneumoniae) and atypical pneumonia (primarily caused by Mycoplasma pneumoniae). Streptococcus pneumoniae is the most common cause of bacterial pneumonia, though other bacteria including Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila, can also contribute. These pathogens infect the lungs, triggering inflammation that leads to symptoms such as cough, fever, and difficulty breathing. The infection typically follows an upper respiratory tract infection or arises from a weakened immune system.
The mechanism of infection generally involves the secretion of cytokines that recruit neutrophils and macrophages to eliminate the pathogen. Additionally, toxins produced by S. pneumoniae and M. pneumoniae can cause cellular damage and exacerbate inflammation. However, vitamin C does not possess direct bactericidal or bacteriostatic properties. Therefore, effective management of bacterial pneumonia requires appropriate antibiotic therapy to eliminate the infection. While vitamin C may help mitigate inflammation-related symptoms, it does not directly target or resolve bacterial infections.
Viral pneumonia, commonly caused by influenza viruses, RSV, and coronaviruses (including SARS-CoV-2), involves direct respiratory tract infection lung inflammation. Symptoms may range from mild to severe and often follow a cold or flu-like illness. Although generally less severe than bacterial pneumonia, viral pneumonia can lead to complications, particularly in the very young, elderly, or immunocompromised individuals. In our study, both vitamin C intervention groups showed a trend towards shorter lengths of stay in viral pneumonia compared to the placebo group, although this was not statistically significant. This suggests that the benefits of vitamin C supplementation in viral pneumonia remain unclear, conflicting with the results reported in previous studies [31,32]. While vitamin C has immunomodulatory effects, including enhanced T-lymphocyte proliferation, improved phagocyte function, interferon production, and viral replication suppression, its overall impact on viral pneumonia outcomes remain uncertain.
In a previous study [33], there was an inverse correlation of vitamin C with the severity of the disease shown, especially in patients presenting with acute respiratory distress syndrome, and who showed a significantly lower vitamin C level. However, in our study vitamin C levels did not show any correlation with the clinical severity of pneumonia. This may be due to the overall clinical severity in our study being mild to moderate with no patients experiencing respiratory failure or acute respiratory distress syndrome. These findings are similar to those in previous studies which showed that vitamin C did not affect the less ill patients [12] and patients with severe viral pneumonia [12,26,30]. Also, in our study, the effect of the vitamin C supplement on patients with higher severity who needed a high-flow nasal cannula showed both a shorter length of stay and a shorter duration of needing the high-flow nasal cannula, but these findings were not statistically significant. Vitamin C supplementation may be beneficial for patients with CAP, through its effects on reducing inflammation and oxidative stress [12,26]. However, further studies are needed to confirm these findings and provide clearer clinical evidence. Current treatment for severe CAP primarily relies on antibiotics (for bacterial infections) and supportive care.
The dosage of vitamin C supplementation has varied across previous studies [12,13,31]. Most past studies administered the vitamin orally, but the research team believes that the route of vitamin C administration may affect drug absorption, especially in patients who have difficulty eating or in children with issues related to taking medication. In our study, we administered the vitamin intravenously and used 2 doses: a low dose of 15 mg/kg/dose intravenously every 6 hours for the low-vit C group, and a higher dose of 30 mg/kg/dose intravenously every 6 hours for the high-vit C group. Both dosages were effective in reducing the clinical severity of the patients; however, we emphasize that the low dosage was particularly effective. These findings represent novel insights, as previous studies have typically used oral administration daily dose [12,13] or focused on adult populations [30,31,34]. There is limited data on the appropriate dosage and duration of vitamin C treatment in pediatric patients. Our study suggests that a low dosage of 15 mg/kg/day, administered every 6 hours for 3 days, can significantly improve clinical symptoms within 48 hours, with minimal side effects.
Adverse side effects were observed in both the high-vit C and low-vit C groups. Two patients, one from each group, experienced mild, self-limited diarrhea, which resolved after discontinuing vitamin C supplementation. This effect is likely due to vitamin C’s osmotic properties; at high concentrations in the intestines, it draws water from surrounding tissues, increasing intestinal water content and potentially leading to diarrhea [35].
Two patients in the high-vit C group developed urine calcium oxalate 3 days after enrollment. Excessive vitamin C intake promotes oxalate crystal formation in the kidneys, potentially increasing the risk of kidney stones, particularly in individuals with predisposing factors (e.g., dehydration or preexisting kidney conditions). This occurs because vitamin C is metabolized into oxalate, which can accumulate in the urine [36]. However, follow-up urinalysis on the seventh day after discontinuing vitamin C supplementation showed normal results in both patients.
This study had some limitations. First of all, the study did not include patients with high severity of pneumonia such as acute respiratory failure and acute respiratory distress syndrome. Secondly, we did not record the effect of vitamin C on the needed duration of oxygen supplementation other than by the high-flow nasal cannula. A further limitation is the use of concomitant therapies, which were determined by the attending physician. These included antibiotics for bacterial pneumonia, bronchodilators for bronchospasm, oxygen supplementation for hypoxemia, and fluids for dehydration. These interventions could have influenced the study's results, potentially prolonging hospital stays or affecting clinical scores. While these confounding variables were beyond our control, we believe that the study outcomes reflect real-world clinical practice, where is often used in conjunction with these other medications. Therefore, we are confident that the results are applicable to settings similar to our hospital.
For future research, we suggest that the following situations be included in the study (1) the effect of vitamin C supplementation on childhood pneumonia with critical severe illness, (2) the effect of vitamin C supplement on each pathogen of viral or bacterial pneumonia, (3) patients with pneumonia who have vitamin C deficiency should be specifically studied to identify the effect of vitamin C supplementation.
In conclusion, vitamin C supplementation for patients with childhood pneumonia requiring hospitalization did not show a reduction in the LOS. However, vitamin C supplementation improved the clinical severity score, with the reduction maximized at 48 hours after treatment. The recommended dosage of 15 mg/kg/dose has been demonstrated to be effective with minimal side effects.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research received foundation from the Faculty of Medicine, Naresuan University, Phitsanulok, Thailand (MD2566C004). The funder had no role in the design, data collection, data analysis, and reporting of this study.
Acknowledgments
The authors would like to thank Mr. Roy I. Morien of the Naresuan University Graduate School for his editing of the grammar, syntax and general English expression in this manuscript. A special acknowledgment also to Miss Daisy Gonzales from the International Relations Section, Faculty of Medicine, Naresuan University for her valuable assistance in editing the manuscript.
Author Contribution
Conceptualization: CP, KS; Data curation: CP, KS; Formal analysis: KJ; Funding acquisition: CP; Methodology: CP; Writing-original draft: CP, KS; Writing - review & editing: CP, KS