Microplastic and human health with focus on pediatric well-being: a comprehensive review and call for future studies
Article information
Abstract
Although humans are highly dependent on plastics from infancy to adolescence, these materials can degrade into ubiquitous microplastics (MPs) that affect individuals at every stage of life. However, information on the sources, mechanisms, detection techniques, and detrimental effects of MPs on children’s health from infancy to adolescence is limited. Hence, here we identified and reviewed original research papers published in 2017–2023 across 11 database categories in PubMed, Google Scholar, Scopus, and Web of Science to improve our understanding of MPs with a focus on pediatric well-being. These studies found that milk and infant formulas are common sources of MP exposure in infants. Infant formula is the dominant source of MPs in babies, while plastic toys are a common source of MPs in toddlers. Adolescents are frequently exposed to MPs through the consumption of food contaminated with MPs and the use of plastics in food packaging. Water and air are sources of MP exposure in children from infancy through adolescence. This study thoroughly summarized how MP exposure in children of all ages causes cell damage and leads to adverse health effects such as cancer. With appropriate authorization from the relevant authorities, small amounts of human biological samples (10 g of feces) were collected from volunteers to assess the amounts of MPs in children with the aim of promoting pediatric well-being. The samples were then treated with Fenton's reagent, stored in glass jars, and filtered through nonplastic filters. Finally, MPs in children were quantified using stereomicroscopy and characterized using micro-Fourier transform infrared spectroscopy.
Key message
· Milk and formula are common sources of microplastic in infants.
· Water and air are the most common sources of micropla stic pollution from infancy to adolescence.
· Microplastic use by children of all ages can cause cell damage and affect their health.
· Microplastics present in children can be quantified using a stereomicroscope and characterized using micro Fourier transform infrared spectroscopy.
Introduction
Parents extensively rely on plastic products for childrearing purposes [1,2]. During infancy, people use plastic bed linens, breast pumps, and diapers [3,4]. When disposed of in the environment, the plastic components of these materials have the potential to break down into microscopic particles (<5.0 mm), known as microplastics (MPs), which carry associated risks to both environments [5,6]. Research on the presence of MPs in the environment and their impact on human health is gaining traction globally, particularly among pediatricians and physicians, with China leading this field (Fig. 1) [7-10].
Over the past 5 years, there has been a considerable increase in the number of publications on the occurrence of MPs and their effects on human health (Fig. 2) [11]. The presence of MP in different human biological systems has been reported in a growing number of studies. For example, MPs have been found in 15 human biological systems, including the blood, liver, lungs, placenta, kidneys, spleen, and sputum [12]. There are several reasons for the presence of MPs in the human biological system. For instance, Feng et al. [13] showed that MPs may occur in the biological components of humans from many sources such as food, drinks, and the air.
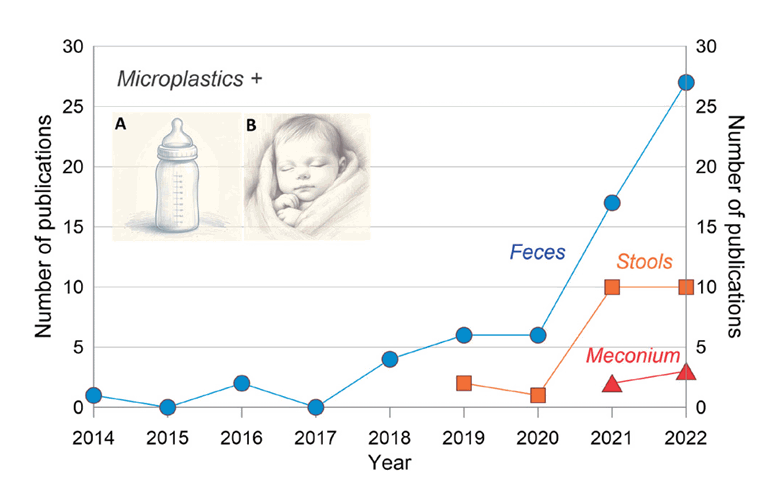
Publications regarding microplastics in human children feces, stool, and meconium over the past years (2014–2022), with (A) baby formula being a dominant source of microplastic in (B) babies over breast milk.
According to Schwab et al. [14], certain age groups are more vulnerable to MPs entering the human body via different paths. For instance, Zhang et al. [15] reported that babies are exposed more to MPs than adults using samples of human excrement from 10 adults and six 1-year-old infants. However, to the best of our knowledge, no study has examined the cause and origin of MPs from infancy through adolescents. Thus, it would be interesting to investigate the most likely sources of MPs and the mechanisms involved in their impact on human development from infancy to adolescence. Such efforts will help protect children's health and aid our understanding of the differences in MP exposure levels among age groups.
Not only have researchers recorded and examined the possible sources of MPs in the human body, but they have performed some preliminary measures to examine the possible impacts of MPs on adults [16-18]. One such study examined how MPs affect the morphology of human-like organisms (Fig. 3). However, the direct effects of MPs on the health of infants, children, and adolescents remain largely unknown. Moreover, despite an increase in studies on the prevalence and effects of MPs in humans, established protocols or studies are lacking that outline the pretreatment and analysis steps for studying MP occurrence in children’s biological components supplied voluntarily by babies, toddlers, and adolescents. A thorough assessment of the methods employed is required to benefit from the study of MPs in children. Therefore, this study aimed to: (1) review recent studies of the sources of MPs in infants, babies, and adolescents and their associated physiological mechanisms; (2) examine the impact of MPs on the health of children from infancy to adolescence; and (3) appraise the techniques of human biological pretreatment and analyze MP identification in children.
Materials and methods
1. Review procedure
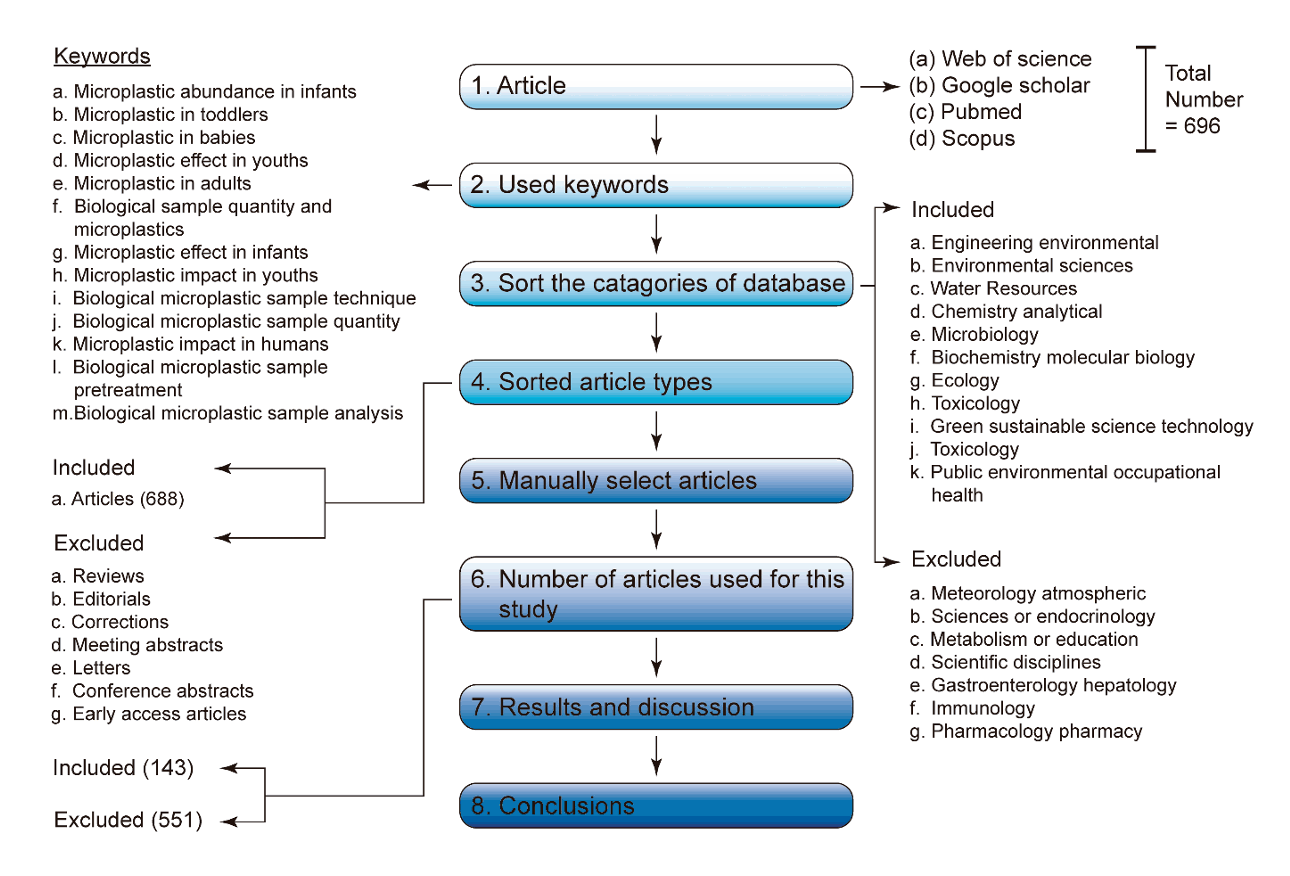
Research articles and peer-reviewed book chapters on MPs and children's health were identified in PubMed, Google Scholar, Scopus, and Web of Science (Fig. 3). Only studies pertaining to MPs and human health were selected using cross-sampling. Chia et al. [19] noted that not every article returned by search engines was guaranteed to meet a study’s objectives; as such, some articles were excluded to ensure fair coverage, objectivity, and accessibility.
2. Selection criteria for manuscripts and data sources
Articles published from 2017 to 2023 were selected using keywords such as “microplastic abundance in infants,” “microplastics in toddlers,” “microplastics in babies,” “microplastic effect in youths,” “microplastic abundance in adults,” “biological sample quantity and microplastics,” “microplastic effects in infants,” “microplastic impact in youths,” “biological microplastic sampling technique,” “biological microplastic sample quantity,” “microplastic impacts in humans,’’ “biological microplastic sample pretreatment,” and “biological microplastic sample analysis.”
A total of 696 articles, including 4 on MP abundance in infants, 1 on MPs in toddlers, 5 on MPs in babies, 1 on MP effects in youth, 148 on MP abundance in adults, 9 on biological sample quantity and MPs, 1 on MP effects in infants, 1 on MP impact in youth, 14 on biological MP sample techniques, 4 on biological MP sample quantity, 449 on MP impacts in humans, 2 on biological MP sample pretreatment, and 57 on biological MP sample analysis, were selected. Among those 696 publications, reviews, editorials, corrections, conference abstracts, letters, conference papers, early access articles, and reviews were eliminated.
All papers underwent a thorough screening process to ensure their validity and eliminate any copies or modified versions of the original research publications. Excluded from the selected papers were studies that did not provide a clear description of the processes used to gather human biological samples to evaluate MP. One hundred forty-three of the 696 papers that were screened were chosen as primary datasets for the study. The 11 database categories that contained the articles used in this study were: “engineering environmental,” “environmental sciences,” “water resources,” “chemistry analytical,” “microbiology,” “biochemistry molecular biology,” “ecology,” “toxicology,” “green sustainable science technology,” “toxicology,” and “public environmental occupational health.
Results and discussion
1. Origin of MPs in children at all stages
From birth, babies, especially those under 1 month of age, rely on the use of plastics for their care and subsistence, including feeding bottles and disposable diapers made of polyester or polyurethane [20]. Through infancy (<1 year old) and later toddlerhood (1–2 years old), children are exposed to additional plastics through the use of toys and other plastic products at home or in daycare facilities [21]. Finally, children use more plastics for several everyday activities, including drinking from a straw, using phones, and using toys as they grow and reach adolescence (12–17 years old) [22]. Therefore, to enhance pediatric well-being and awareness, it is essential to have a complete and updated understanding of the ways in which children become exposed to MPs from infancy to adolescence.
1) Origin of MPs in babies, infants, and toddlers
According to recent studies, MPs in babies, infants, and toddlers originate from various sources (Table 1). Breast milk and baby formula are the main sources of MP exposure in infants and toddlers. Milk is a baby's primary dietary source until toddlerhood because it offers a good assortment of well-balanced nutrients that are necessary for growth and development [23,24]. Infant formula was developed to supply the nutrients required for infants' growth and development [25,26]. Formula milk can be a substitute for breast milk [27,28]. Breast milk is generally accepted as the ideal food source during infancy [29,30].
Research has shown that breastfed infants have lower MP exposure levels than formula-fed infants. According to Liu et al. [31] and Ragusa et al. [32] MPs in infant formula account for as much as 11 items/g of exposure in infants. This exceeded the exposure of breastfed infants (2.72 items/g). Breastfed babies are less likely to be exposed to MPs than formula-fed babies for several reasons. First, infants and/or toddlers are more susceptible to MP exposure from the materials used in the manufacturing and packaging of infant formulas than from breast milk [33,34]. Unlike breast milk, which does not require these materials before ingestion, items such as the water and cooking utensils used to prepare infant formulas at home before feeding may be teeming with MPs [8,35,36]. For example, MPs from the tap water used to dissolve powdered milk contaminate the milk. Kim et al. [37] and Li et al. [38] asserted that approximately 72.32 items/L MPs contaminate home tap water, bottles, and ground water.
Second, the bottles or cups used to administer milk or water to children are another source of MPs in babies, infants, and toddlers. The plastic polymers used to make the majority of baby cups and bottles, such as polypropylene and/or polyethylene, can break down into MPs [4,39]. As reported by Li et al. [38] baby bottles may emit 16,200 MPs/L. For instance, Chen et al. [40] revealed that children's use of plastic cups may be responsible for approximately 781–4,951 particles/L.
Third, babies and young children may come in contact with MP through masks made of plastic polymers, such as polypropylene, for medical and pediatric use. If these masks are exposed to environmental factors, including heat, moisture, and direct sunlight before or after use, they may eventually degrade into MPs [41,42]. For instance, baby masks can discharge 1.55 items/mask into the air, which babies, infants, and toddlers can breathe in and internalize [42].
Finally, according to Chia et al. [43] Another source of MP exposure is the air in the homes of babies, infants, and toddlers. In addition to via milk, the most common direct source of MP in babies, infants, and toddlers, MPs can enter the bodies of these age groups through airborne exposure. For instance, Zhu et al. [44] found that home air contained approximately 1,174 particles/g, which may increase children's exposure to MP. Perera et al. [45] stated that the air in childcare centers may have a particle content of 2.25 ppm.
2) Origin of MPs in adolescence
Many items that adolescents use on a regular basis, such as phones, meals, snacks, drinks, and personal hygiene products, are packaged in plastic. Compared to other materials, their affordability, ease of use for everyday tasks, and versatility make them a reasonable option for financially challenged adolescents. Several recent studies have demonstrated that adolescents who use these devices or consume plastic-packaged food or that grown in MP-contaminated soil may be exposed to MPs (Table 2) in various ways.
First, teenagers who use plastic food containers and utensils as well as those who eat food grown in soil contaminated with MPs can gradually accumulate MP in their bodies as a result of their decomposition and entry into the body (Fig. 4) [46,47]. For instance, extruded polystyrene and polystyrene food containers have been demonstrated to entangle food [48]. Du et al. [49] revealed that food carried in plastic takeaway meal packages could be contaminated with approximately 29 items/g of MPs. Therefore, teens may be exposed when consuming such food. Zhou et al. [50] revealed that food packaging containers could produce 29–552 items/g of MPs. Therefore, eating food stored in containers with attached MPs may result in MP ingestion.
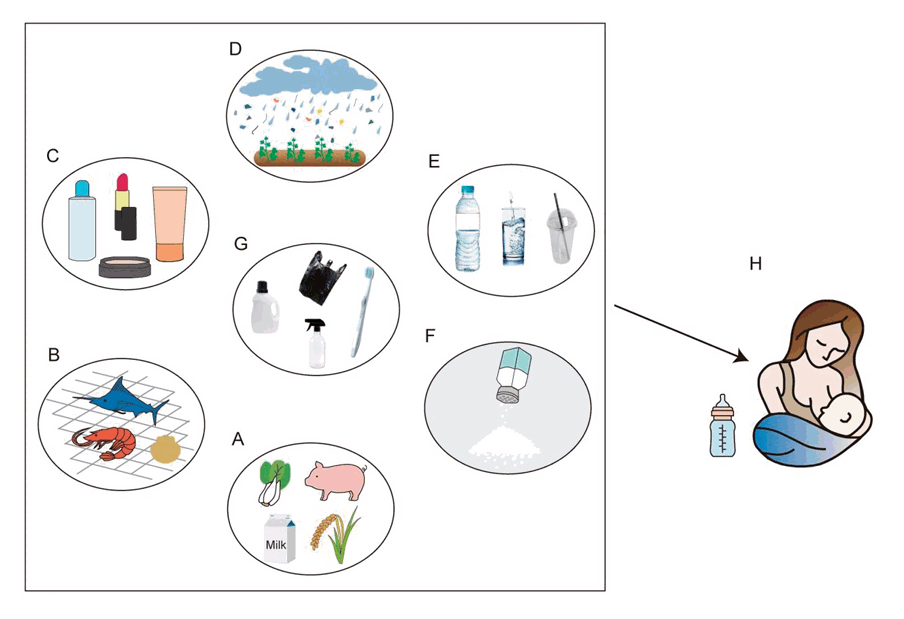
Sources of microplastics exposure in children from (A) food, such as milk, meat or vegetables; (B) crayfish and other seafood; (C) breast milk; (D) air; (E) plastic cups, bottled water; (F) sea salt (G) plastic packaging materials; (H) breast milk contaminated with microplastics.
Second, adolescents were more likely to be exposed to MP when eating seafood (Table 2). Studies have indicated that marine MPs contaminate seafood [51,52]. According to Park et al. [53], each fish can be contaminated with 5–56 MPs. In addition, a single crayfish can contain 0.17–0.92 MPs [54]. As crayfish are a staple element in many coastal cuisines (Fig. 4), people living in coastal areas may have high accumulation of MPs. Consequently, MPs can enter the bodies of children who consume affected crayfish. Interestingly, fish from Antarctica reportedly exhibited higher MP levels [55].
Third, teens can be exposed to MP in tap or bottled drinking water. Cha et al. [56] and Chia et al. [57] recently reported that MPs contaminate groundwater, a source of drinking water in many countries. Thus, drinking groundwater can expose adolescents to MPs.
Fourth, food cooked with salt-containing MPs is another source of MP exposure (Table 2). Teens may consume MPs via foods that contain MPs and are heavy in salt. For instance, Lee et al. [58] demonstrated that cooking with table salt can result in the occurrence of 9.77 MPs/kg of food.
Fifth, air may be a source of MP exposure. Teens may be unintentionally exposed to atmospheric MPs. For instance, Perera et al. [45] stated that the air in an office area alone could contribute to approximately 1.2 particles/m3 of air. Varying the conditions produced different concentrations of MPs. Liao et al. [59] reported that the contributions of MPs from urban and rural air were 224 and 101 particles/m3 of air, respectively.
2. Effects of MP exposure on infants’, toddlers’, and adolescents’ well-being
Breathing clean air, eating a balanced diet, and drinking clean water are necessary to maintaining good health in children and adolescents [60]. However, if the food, water, or air is contaminated, newborns, babies, toddlers, and teens may be at risk of health issues [61,62]. Experts, notably medical professionals, are increasingly worried about the health of children and adolescents pertaining to MP exposure through food (seafood), water, and air [63]. A few studies have demonstrated that the presence of MPs in the body can lead to toxicity (Table 3) [60,64]. The following mechanisms are related to the toxicity and harmful effects of MPs in newborns, babies, toddlers, and teens.
First, mitochondrial damage can occur in children and adolescents with MP accumulation, potentially resulting in cancer [60,64]. The concept that animal cells exposed to polystyrene MPs may experience mitochondrial damage, ultimately resulting in epithelial ovarian cancer tumors in mice, is supported by a study by Chen et al. [65]. Although it is unethical to use additional cells to assess the effects of MPs on human cells, our results may be applicable to humans.
Second, by altering hepatic metabolites and increasing serum alanine transaminase (ALT) and aspartate transaminase (AST) levels, MP exposure in children and teens could harm hepatocytes [66,67]. When hepatocytes that normally contain these enzymes are damaged, ALT and AST are released into the bloodstream [68,69]. For example, Ge et al. [70] demonstrated that MPs are hepatotoxic. Therefore, if MPs are consumed in large amounts, blood tests that are frequently used to identify liver injury may reveal elevated ALT levels as a sign of liver damage.
Third, MPs can contribute to oxidative stress in children and adolescents by creating an imbalance between the amounts of free radicals and antioxidants in the body. Xiong et al. [71] showed that exposure to polystyrene MPs may result in oxidative stress in the human body, which deteriorates kidney function. Moreover, oxidative stress can harm cells and is associated with kidney disease [72,73].
Fourth, research suggests that MPs may become lodged in the respiratory tracts of children and teens, causing inflammation within the respiratory system. For example, MPs in the human respiratory system can alter cell survival, trigger the transcription of inflammatory genes, and alter the expression of cell cycle–associated proteins [74]. Stimulation of inflammatory gene transcription by the respiratory system can result in various respiratory diseases, such as asthma, bronchitis, and chronic obstructive pulmonary disease. Breathing problems are caused by inflammation-induced tissue damage, mucus production, or airway constriction.
Finally, research has shown that polystyrene MP exposure in rats changes the endocrine system, including levels of luteinizing hormone and follicle-stimulating hormone, and contributes to other endocrine system disorders that potentially affect growth and reproduction [75,76]. Moreover, mammalian experiments and reviews have shown that MPs consist of endocrine-disrupting chemicals such as bisphenols and phthalates that can disrupt the immune system [76,77]. Therefore, adolescents exposed to MPs may experience endocrine changes in adulthood. It is difficult for girls to reach adulthood and create viable eggs for fertilization if MP exposure causes irregular or non existent ovulation. In the absence of ovulation, few or no viable embryos can form. MPs hinder embryonic growth and reduce the likelihood of pregnancy. Wei et al. [78] observed that mice exposed to polystyrene MPs had a reduced conception rate and generated fewer embryos than their unexposed counterparts.
3. Biological sample collection and pretreatment for MP analysis in individuals
1) Perquisites to biological MP sample collection
Despite MP exposure in humans, there is currently no recognized protocol for obtaining biological samples to detect their presence in children and evaluate the potential associated health risks. However, to assess the presence of MPs in children, it is crucial that samples be taken from children’s waste materials or organs that are representative of the body. Ethical measures should be followed during the collection of such samples, which should be readily available and sufficient to represent the sources of MPs in children [79].
First, to ascertain the presence of MPs in the bodies of children, it is imperative that samples be obtained ethically from willing participants. This recommended strategy has been extensively reported in studies that collected specimens from adult participants to investigate the occurrence of MPs. For example, Schwabl et al. [14] conducted a study of the occurrence of MPs in adults from Tokyo, Japan, Russia, the Netherlands, the United Kingdom, Italy, Poland, Finland, and Vienna for which 8 people aged 33–65 years volunteered to provide fresh fecal samples.
Second, before samples were collected from children, parents were administered a questionnaire. The survey responses will aid our understanding of the lifestyles of children and their parents and the sources of MPs in children. For instance, in the study by Yan et al. [80] Prior to collecting fecal samples from the study volunteers to examine the presence of MPs in adults, each participant completed a 5-minute questionnaire on their living situation, work status, and eating and drinking habits during the past year.
Third, the researchers must obtain ethical approval from the relevant authorities in each country. For example, a study of young men’s feces conducted in China was approved by the Peking University Institutional Review Committee. Researchers should seek a review of the measurement method standards and follow-up method ethics from their respective institutional review boards [81].
2) Sample quantity required for MP analysis in children
To study MP occurrence in children, the number of specimens required varies depending on the age range of the intended participants, study objective, and laboratory apparatus used to detect them. It is advisable to collect very small numbers of specimens to reduce or eliminate any potential risks. For instance, to study the occurrence of MP in newborns, only approximately 50 g of placenta can be collected 10 min after delivery from the new mother and stored in a glass or metal box for subsequent analysis (Table 4) [82]. In addition, approximately 10 mL of blood and 10 g of fecal samples can be collected to study the occurrence of MPs in children (Table 4). The bulk of current research on MPs and health reports state that feces, as opposed to organs such as the blood, liver, or placenta, are collected for MP examinations (Table 4). This may be explained by the fact that stool sample collection is easy and features a low risk of MP exposure; moreover, no bodily incisions are required to collect waste samples.
4. Specimen storage
Prior to being prepped for MP analysis, collected specimens can be kept in glass containers (Table 4). For instance, according to Leslie et al. [83] and Yang et al. [84], blood was drawn and stored in glass bottles before the analysis of MPs in blood samples. Furthermore, Yan et al. [85] and Zhang et al. [15] stool samples collected for MPs analysis were stored in glass containers. Chia et al. [86] and Cha et al. [87] suggested that eliminating the use of plastic containers could reduce the risk of MP exposure via equipment contaminating samples. So, samples should be kept in nonplastic containers at -20°C to -80°C [81,88] to inhibit the growth of bacteria or fungi and acceleration of MP degradation.
5. Sample pretreatment
The collected specimens (e.g., 5.0 g of feces) were dried in the laboratory for 50–240 minutes at 30°C–50°C [89]. The mixture was then placed in a 1-L glass beaker containing 30% H2O2 or another digestive agent (Table 4). This digesting agent is used to eliminate organic molecules in the sample that could be misinterpreted as MPs. Chia et al. [86] reported that H2O2 is the most effective digestive agent for the decomposition of organic molecules. The digested beaker was submerged in an ice-water bath to prevent high temperatures caused by H2O2, which may damage the MPs [90].
Although H2O2 is the most effective agent for digesting organic molecules, most studies on the occurrence of MPs in humans recommend the use of Fenton's reagent, which is a mixture of H2O2 and Fe2+ (Table 4) [80]. Fenton's reagents can more accurately target specific chemical components in complex matrices such as human specimens, thus minimizing interference from other compounds present in the sample [91].
6. Sample filtration
Prior to the analysis, sample filtering was performed after the specimens collected from the children were prepared, impurities were sorted and removed, and any organic contaminants that may have been confused with MPs during the analysis were chemically digested. A filter paper with a sufficiently large pore diameter was used to collect the minimum MP particle size required during filtering. Furthermore, the samples were not contaminated by the filters [86,92]. To prevent MP contamination or misidentification, nonplastic filters such as glass or gold filters should be used, whereas plastic, paper, and cellulose fiber filters should be avoided [86,93]. Each experiment was conducted in a laminar-flow hood to prevent contamination [94,95].
7. Visualization and quantification of biological MP sample from filters
Once the specimens were filtered to separate them from the solute matrix, the MPs were assessed using the filter membranes employed in the filtration procedure. First, research has indicated that MPs in human specimens (>50 μm) removed from the filters may be visualized with optical instruments such as a microscope. For example, to examine the occurrence of MPs in stool samples, a microscope was used to visually analyze the filter paper following MP pretreatment [80].
Second, MP characteristics can be evaluated using Fourier transform infrared (FTIR) spectroscopy because of its low error rate and increased dependability [96,97]. Nonetheless, the most recent study indicated that MPs can be detected using Raman microscopy instead of FTIR (Table 4). This may be partly because the sample preparation is less demanding for the former than the latter. Moreover, it may be necessary to take extra precautions when using FTIR to ensure that the sample is transparent to infrared light. It is also possible to investigate opaque materials with minimal preparation using Raman spectroscopy [80,98].
8. Methods of evaluating MPs in large human populations
1) Proxy markers of human MP exposure
According to Li et al. [99] epidemiological studies are an effective way to assess and correlate the effects of MP exposure in humans. However, only a few epidemiological studies have focused on MP exposure and human health. The need for a comprehensive and in-depth data collection process makes it difficult to study MP exposure in large populations. However, the use of proxy markers can solve this problem by offering indirect measurements that can indicate MP exposure with poorer resolution but better feasibility for large-scale investigations. In the context of human MP exposure, proxy markers may include the following.
(1) Biomarkers for measuring MPs exposure
Biomarkers used as proxy markers to study human exposure to MPs include certain chemicals or particles detected in biological samples, such as blood or urine, that are associated with MP exposure [63,100,101]. It is worth noting that urine samples can produce more meaningful results than blood samples. Hence, urine biomarkers are preferred [102,103]. According to Menzel et al. [104], a good biomarker should have a suitable half-life, be stable, not be affected by conditions during collection or storage, and be measurable using noninvasive or minimally invasive methods. For example, elevated levels of bisphenol A, a common plastic additive, have been used as a proxy marker for plastic exposure [105-107]. Analytical methods such as mass spectrometry and chromatography can be employed to identify these biomarkers; despite some limitations in specificity, they still provide valuable insights [83,108-110].
(2) Environmental indicators for measuring MPs exposure
Proxy markers classified as environmental indicators include measuring MP levels in environmental matrices, such as water, soil, air, and food, to infer human exposure to MPs [99]. For instance, to measure MP levels in environmental matrices, such as water, it is recommended that approximately 300–500 groundwater samples be collected from each sampling site and sent to a laboratory for analysis [56]. It is worth noting that MP levels in food can be measured indirectly by quantifying the MP levels of the soil where the food is grown, as MPs in the soil eventually enter crops during harvesting. Soil samples as small as 15.0 g were collected to infer the MP levels in the soil environmental matrix [96]. Other studies reported that human exposure to MPs in an environmental matrix can be evaluated through modeling [110-112]. For example, the presence of MPs in drinking water sources can serve as an indicator of their potential ingestion by the population [113,114].
(3) Behavioral indicators for quantifying MPs exposure
KA Surveys that capture dietary habits, the use of plastic products, and residences in MP-polluted areas can help estimate human MP exposure levels [115,116]. For example, Kai et al. [117] requested that each participant complete a 5-min questionnaire about their eating and drinking habits, living habits, and working situations from the previous year to measure the level of human exposure to MPs.
In the study by Zhang et al. [118] each participant was given questionnaires to track their food and water intake every 24 hours over 1–2 weeks to assess their MP exposure levels. Such behavioral data, when linked to MP exposure, could provide a practical data collection approach for large epidemiological studies.
Overall, the integration of proxy markers in epidemiological research offers a practical, cost-effective, and feasible approach to assessing MP exposure across large populations. This methodology both enhances feasibility and provides a more comprehensive understanding and monitoring of MP exposure in human health studies.
Conclusion and future perspectives
Owing to the heavy reliance on plastics for daily life, MP occurrence rates in children are constantly increasing. This study investigated the frequency of MP exposure in infants, toddlers, and adolescents. Food, air quality, and use of plastic devices are possible causes of MP exposure in children. However, this study demonstrated that the incidence of MPs in children varied among age groups. For example, the main sources of MP exposure are milk in infants, plastic toys in toddlers, and seafood in teens. In all age groups, the most common sources of MP exposure were water and air. This study examined how MP exposure in newborns, children, and teens could damage cellular components (such as mitochondria), leading to health problems (including liver damage and cancer). This study also showed that, to evaluate MP quantity in children with the goal of promoting pediatric well-being, human specimens should be frequently obtained from volunteers with permission from relevant authorities. The samples were then subjected to the Fenton process and stored in glass jars. Finally, although this study adds to the field's understanding of the subject, further research is required to address the gaps in our understanding of pediatric well-being and MP pollution in children. The following future studies are required:
(1) Long-term health effects of MP exposure in children are not well understood. Further research is required to assess the cumulative effects over time.
(2) Further research is required to ensure that MPs in infants, toddlers, and teens are measured holistically from various sources including hospitals and post-hospital indoor care facilities, textiles, disposable diapers, wet baby wipes, and personal care products. It would be interesting for research to demonstrate the extent to which these sources affect children's bodies. We were unable to find any relevant studies published to date.
(3) Studies on the long-term health effects of MP exposure in children remain in their infancy. Further investigations are required to fully understand the toxicity and inflammation associated with MP ingestion.
(4) To the best of our knowledge, no studies to date have examined the mitigation of MP in children. Future studies on this subject will be fascinating and aid our understanding of MP exposure in young people.
(5) Several studies have demonstrated that polyethersulfone and polyphenylsulfone are the most prevalent forms of MPs in the environment. To the best of our knowledge, the effects of polyethersulfone and polyphenylsulfone on the human body should be examined as most studies to date have evaluated the effects of MPs on the body using polyphenylsulfone only.
(6) To our knowledge, the field of carcinogenesis is the most sophisticated method for evaluating the effects of substances on human health. It is important to note that the International Agency for Research on Cancer bases its evidence framework in this area of carcinogenesis on 3 main pillars: (i) human epidemiologic evidence, (ii) animal experimental evidence, and (iii) cellular research mechanisms. However, epidemiological data are scarce, and the majority of research to date, based on animal and cell experiments, supports the idea that MP exposure may be dangerous. Further research is required to address this knowledge gap.
(7) Marfella et al. [119] recently showed that MPs can induce vascular damage, a major mechanism of cardiovascular disease in elderly individuals. Therefore, further studies are required to verify whether this is applicable to children.
Notes
Conflicts of interest
No potential conflict of interest rele vant to this article was reported.
Funding
This work was supported by the Korea Environ mental Industry Technology Institute (KEITI) through the Measurement and Risk Assessment Pro gram for Management of Microplastics Program, funded by the Korea Ministry of Environment (MOE) (2020003110010), and this research was supported by the Basic Science Research Program through the National Research Foun dation of Korea (NRF), funded by the Ministry of Education (No.2019R1A6A1A03033167). This study was supported by the Kangwon National University Postdoctoral Position Funded Project (No. C10152280120210402).
Author contribution
Conceptualization: RWC; Data cura tion: RWC; Formal analysis: RWC; Funding acquisition: JYL; Project administration: JYL; Visualization: JC; Writing original draft: RWC; Writingreview & editing: RWC, JYL, VAN, JC