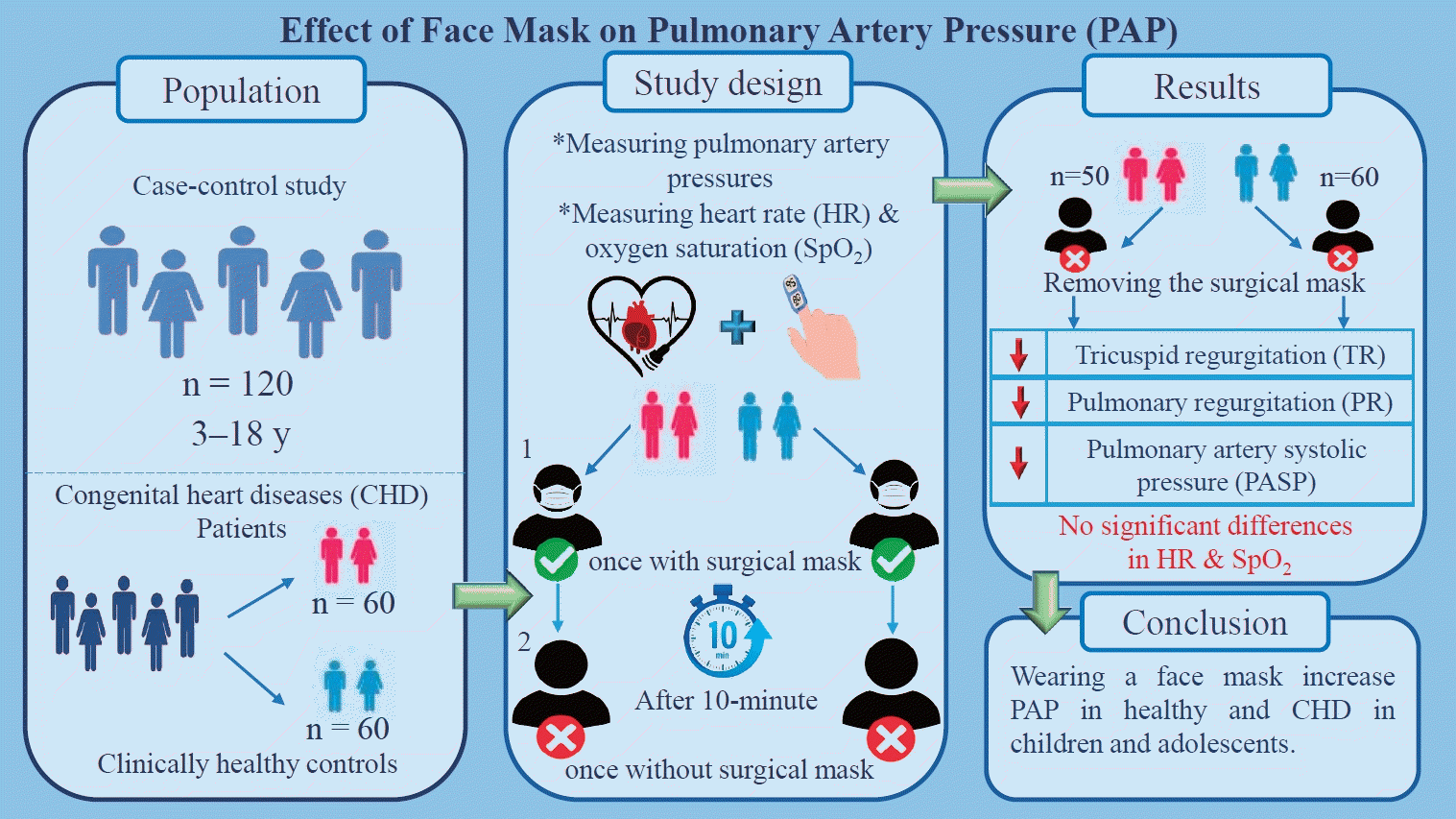
Effect of face mask on pulmonary artery pressure during echocardiography in children and adolescents
Article information
Abstract
Background
Face masks have become an important tool for preventing the spread of respiratory diseases. However, we hypothesized that face masks with reduced nasal airflow may alter pulmonary artery systolic pressure (PASP).
Purpose
This study aimed to evaluate the effect of face masks on PASP in children and adolescents.
Methods
This case-control study was conducted between March 2021 and April 2022 at the Pediatric Cardiovascular Research Center in Isfahan, Iran. Using a convenience sampling method, a total of 120 children and adolescents, boys and girls aged 3–18 years, were allocated into 2 groups of 60 each (case group with congenital heart disease (CHD), control group of healthy subjects). For each patient in the case and control groups, echocardiography (ECHO), heart rate (HR), and blood oxygen saturation (SpO2) were performed and measured twice—once with a surgical mask and once without a surgical mask—by a pediatric cardiologist at 10-min intervals.
Results
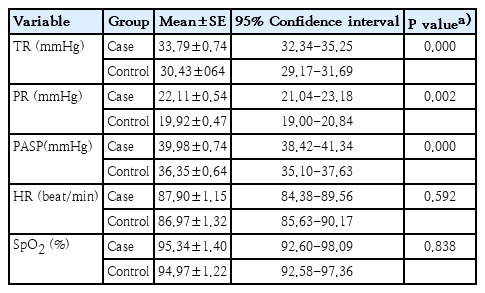
A total of 110 participants were analyzed. The mean patient age was 9.58±3.40 years versus 10.20±4.15 years in the case (n=50) and control (n=60) groups, respectively. Approximately 76.0% (n=38) of the participants in the case group versus 60.0% of those in the control group were male. In the case and control groups, there was a statistically significant reduction in the mean changes in tricuspid regurgitation (P=0.001), pulmonary regurgitation (P=0.002), and PASP (P=0.001) after face mask removal. Although this study showed a reduction in pulmonary arterial pressure after face mask removal in patients with CHD and healthy subjects, no significant changes in HR (P=0.535) or SpO2 (P=0.741) were observed in either group.
Conclusion
Wearing a face mask increased PASP in healthy children and adolescents with CHD; however, the SPO2 and HR remained unchanged. Therefore, mask removal during ECHO is recommended.
Key message
Question: Can face masks alter pulmonary pressure in children and adolescents with and without congenital heart disease?
Finding: Mask removal during echocardiography (ECHO) reduced pulmonary pressure.
Meaning: These findings suggest that face masks should be removed during ECHO in children and adolescents.
Graphical abstract.
Introduction
The World Health Organization (WHO) on March 11, 2020, declared the outbreak of a public health emergency of international concern due to the novel virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019, COVID-19) [1]. Globally, as of 8 June 2023, a total of 767,750,853 confirmed cases of COVID-19, including 6,941,095 deaths, have been reported to WHO [2]. Reported cases of COVID-19 among children and adolescents increased dramatically in the year 2022 during the rise of the Omicron variant and its various sublineages. For example, up to July 24, 2022, children under 5 and those aged 5 to 24 years old accounted for 2.47% and 24.35% of COVID-19 cases, respectively [3]. across the world. The rapid spread of the virus affected people’s lives all over the world [4]. Many governments were forced to take urgent measures, including social distancing, frequent hand washing, and the use of face masks, to delay the spread of the disease and prevent further infection [4,5]. Based on the reports from WHO, so far no vaccine has been 100% effective, therefore, one way to protect the health of children and adolescents who are not currently eligible for vaccination is hand washing and wearing face masks. The use of face masks is a clinically accepted method for reducing the transmission of coronavirus through droplets and contact routes [5]. After the outbreak of SARS-CoV-2 pandemic, the wearing of masks in public spaces has been widely recommended by government authorities [6]. Whilst the use of face masks is an understandably rational strategy to prevent and reduce coronavirus transmission, there exist some concerns as to whether wearing a face mask would be a deleterious physiologic burden to the human body [7]. Some research findings show that the use of face masks could impact human physiological functions, such as pulmonary artery pressure, heart rate (HR), increased airway resistance, carbon dioxide retention, hypoxia, and other lung function changes, leading to increased heart load [8]. Pulmonary artery hypertension (PAH) is a significant cause of morbidity and mortality in children and adolescents [9]. PAH is a pathological hemodynamic condition that is defined as an increase in mean pulmonary artery pressure (m PAP)≥20mmHg at rest, and is assessed by right heart catheterization (RHC) or echocardiography (ECHO) and can complicate the majority of cardiovascular and respiratory diseases [9,10]. PAH is divided into primary and secondary forms [11]. Primary pulmonary hypertension is a disease of unknown etiology, and secondary pulmonary arterial hypertension is due to an adverse outcome of a variety of systemic disorders, such as chronic obstructive pulmonary disease, collagen vascular diseases, congenital heart diseases (CHDs), heart failure, low oxygen levels in the blood for a long time, and other diseases [11,12]. Moreover, based on the results of studies, stenosis developing in the upper respiratory tract as a result of different causes may lead to obstructive increased pulmonary artery pressure [13]. Results of the study by Lima et al. [14] show that diseases, such as adenotonsillar hyperplasia in children can cause pulmonary vasoconstriction and pulmonary hypertension by affecting the upper airways. A very common form of PAH diagnosed in childhood and adolescence is associated with CHD [15]. RHC is the gold standard for the diagnosis of PAH. However, due to its high cost and risk, ECHO with good sensitivity and specificity, is now a safe, readily available, and less expensive alternative to catheterization for pulmonary artery pressure detection [16]. The results of other studies show that, so far, the effect of face masks on pulmonary artery pressure has not been evaluated by ECHO in children and adolescents with CHD. An earlier evaluation by our researchers in this study showed that during ECHO, pulmonary artery pressure might be increased in CHD patients who have used a face mask. Therefore, it was hypothesized that the use of face masks might have a positive effect on pulmonary artery pressure in all children and adolescents, especially in patients with CHD. This study sought to clarify whether wearing a face mask in children and adolescents, especially with CHD can cause pulmonary vasoconstriction and increase pulmonary artery pressure by affecting the upper airways.
Methods
1. Study design
This case-control study was conducted from March 2021 to April 2022 at the Pediatric Cardiovascular Research Center in Isfahan, Iran. The target population consisted of 120 children and adolescents aged 3–18 years. By using the convenience sampling method, the sample was selected among children and adolescents who were referred to a pediatric cardiology clinic for evaluation of heart diseases and diagnosed with CHD. Control subjects were children and adolescents who had been referred to a pediatric cardiologist for a reason other than CHD and were matched for age and gender with the case group.
The protocol was approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED. REC.1399.1123), and all participants signed the written informed consent before participation in the study.
2. Sample size calculation
The sample size for the present study was calculated based on similar studies [17]. The confidence interval was 95.0 %, while the study power (1-β) was set at 85.0% and the relative error was 5%. The final sample size for both groups was about 120 (n=60 per group).
3. Participants
A total of 120 children and adolescents (aged 3 to 18 years) took part in this study (case group; n=60, control group; n=60). The children and adolescents in the case group consisted of noncyanotic CHD patients, without the need for medication, and were examined by a pediatric cardiologist. The control group consisted of children and adolescents who presented for cardiac assessment, such as chest pain and showed no evidence of CHD in the ECHO done for them at the pediatric cardiology clinic.
Individuals with Down syndrome, symptoms and signs of obstruction of the upper respiratory tract, respiratory infections, emphysema, pulmonary fibrosis, adenoid hypertrophy, asthma, sleep apnea, oral and facial anomalies, and those using medication(s), as well as participants and parents who did not cooperate to perform the second-stage ECHO were excluded from this study.
4. Measurements
This study was conducted to investigate the effect of surgical masks on pulmonary artery pressure using Doppler ECHO.
Participants' demographics (including age, sex, weight, height, and time of pre-ECHO face mask use) were recorded in both groups after obtaining written consent informed from all parents using a checklist.
At first, due to the outbreak of coronavirus disease, all participants in case and control groups (COVID-19) were requested to have surgical face masks before doing the first ECHO. For each patient in the case and control group,ECHO was performed and HR, and oxygen saturation (SpO2) were measured twice once with and once without surgical mask by a pediatric cardiologist at 10-minute intervals [17]. It is noteworthy that the pulmonary artery pressures of all participants in both groups in supine and resting positions were measured with an echocardiographic probe (HS70A, Samsung Healthcare, Seoul, Korea) by a pediatric cardiologist as follows Fig. 1:
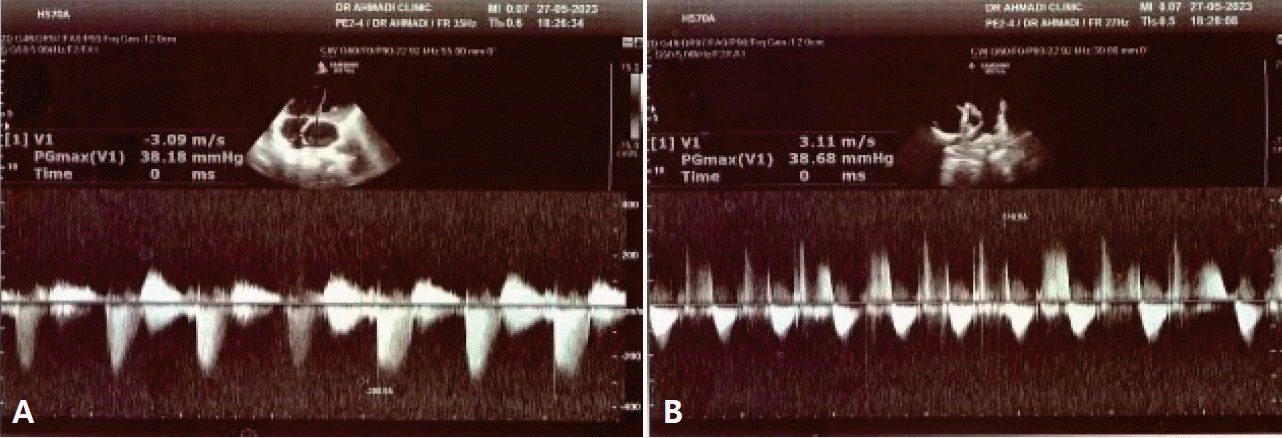
(A) Tricuspid regurgitation velocity and gradient. (B) Pulmonary regurgitation velocity and gradient.
(1) The difference in pressures between the right ventricle and right atrium was measured through continuous wave (CW) Doppler of the tricuspid regurgitation (TR) trace in the 4-chamber view. The simplified Bernoulli equation (P=4 [TR max]2) was then used to calculate this pressure difference using peak TR velocity. To obtain a satisfactory envelope, CW Doppler was used at a sweep speed of 100 mm/sec [18].
(2) Pulmonary regurgitation (PR) was measured in the parasternal short-axis view using CW Doppler. The beginning of the PR signal was measured; the beginning of the PR signal is the peak PR velocity [18].
(3) Pulmonary artery systolic pressure (PASP) was estimated by adding the peak velocity of the envelope (TR max) to right atrial pressure which is assumed by the size and distensibility of inferior vena cava during inspiration at rest.8)
Using pulse oximetry (CMS50D1, Lot: 80.20537, Contec Medical Systems Co., Ltd., Qinhuangdao, China), SpO2 and HR were measured for all participants wearing a surgical mask. After collecting the information of the first stage in the designed checklist, the participants and one of their parents were asked to wait for 10 minutes without a mask in a room with a suitable ventilation system and no access to other people. After 10 minutes, TR/PR and PASP pulmonary artery pressure were measured again and recorded through ECHO. It should be noted that in the present study, due to the cooperation of the children, sedative drugs were not used.
SpO2 and HR of participants were measured by pulse oximetry without the use of a mask by a pediatric cardiologist. In addition, the height and weight of children and adolescents were measured with minimal clothing and no shoes. The height was recorded with an accuracy of 0.5 cm. The weight was also measured using a scale (786 Mechanical column scale, Seca, Hamburg, Germany) with an accuracy of 0.1 kg.
5. Statistical analysis
The data collected were analyzed using IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA), descriptive statistical tests, and inferential statistics including independent t tests, analysis of covariance (ANCOVA), paired t tests, and estimated marginal test. The Kolmogorov-Smirnov test was used to check the normality of the data and the level of significance was defined as P<0.05.
Results
During the study, out of 120 participants (3–18 years), 10 patients and their parents were excluded from the case group because they refused to perform the second stage of ECHO. In this study, the mean ages of the children and adolescents in the case and control groups were 9.58±3.40 and 10.20±4.15 years, respectively. The mean weight of the participants was 31.19±16.23 and 34.47±15.45 in the case and control groups, respectively. About 76.0% (n=38) of participants in the case group and 60.0% in the control group were male.
The most common types of CHD in children and adolescents in the case group were atrial septal defect (38.0%, n=19), ventricular septal defect (26.0%, n=13), and patent ductus arteriosus (10.0%, n=5).
The mean duration of face mask before performing ECHO was 41.70±26.21 minutes versus 46.83±26.33 minutes in the case and control groups, respectively. According to the results presented in Table 1, there was no significant difference between the 2 groups in terms of demographic characteristics before the intervention.

Patients’ demographic characteristics of case (with congenital heart disease) versus control (healthy subjects) groups (N=110)
Independent sample t test results indicated no statistically significant differences (except for HR and SpO2) between the case and control groups in the mean gradient of TR (P=0.611), mean gradient of PR (P=0.106) and mean of PASP (P=0.640).
The paired t test results showed a significant difference between the mean gradient of TR, PR, PASP, and HR of all participants in case and control groups with and without masks (P<0.05), but there was no significant difference in SpO2 between case groups (P=0.566) (Table 1).
Differences between the case and control groups in posttest mean for TR, PR, PASP, HR, and SpO2 were compared by ANCOVA, controlling for pretest mean.
In Table 2, ANCOVA results revealed the significant differences between the mean changes of TR (F=11.554, P=0.001), PR (F=9.677, P=0.002), and PASP (F=12.714, P=0.001) of participants in the case and control groups after adjusting for the covariates. No significant difference was observed between the mean changes in HR (F=0.387, P=0.535) and SpO2 (F=0.110, P=0.741) in the case and control groups (Table 2).
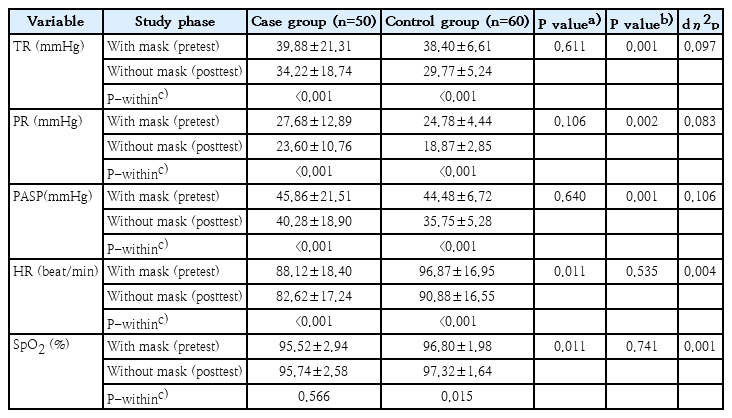
Mean study variables of case (with congenital heart disease) versus control (healthy subjects) groups (N=110)
Inspection of the estimated marginal means shown in Table 3 indicated that children and adolescents with CHD (without mask) had higher adjusted mean scores for TR, PR, and PASP than the clinically healthy control subjects (P<0.05). When adjusted from age, sex and baseline values (With mask). This result provides strong support for the other results of this study (Table 3).
Discussion
After the pandemic coronavirus (COVID-19), the use of face masks was recommended by WHO to prevent the spread of the virus [18]. We observed that children and adolescents with face masks referred to a pediatric cardiology clinic for evaluation of heart diseases had higher pulmonary artery pressure during ECHO. Therefore, for the authors of the present paper, the question was: whether a face mask affects pulmonary artery pressure, HR, and SpO2.
To our knowledge, this is the first study to survey the effect of face masks on pulmonary artery pressure using ECHO in children and adolescents with CHD and healthy subjects.
The results of ANCOVA revealed that there were significant differences in the mean changes of TR, PR, and PASP between the case and control groups. In the current study, the ECHO of patients who removed face masks showed that the mean TR gradient decreased in the case group (5.65 mmHg) and the control group (8.63 mmHg). Additionally, we noticed a decrease in the PR gradient for the case group (4.08 mmHg) and the control group (5.91 mmHg). In the case and control groups, the reduction in the mean of PASP was reported to be 5.58 mmHg and 8.71 mmHg, respectively.
In this direction, Ferreira et al. [18] conducted a study with the aim of identifying the relationship between PASP and nasal obstruction in children through Doppler ECHO. Findings of their study showed that the reduced nasal airflow and upper airway obstruction may increase PASP.
In addition, the research results showed that the face mask function is partially determined by the resistance of the airflow provided. It has been considered possible that resistance to airflow could negatively impact pulmonary gas exchange and heighten pulmonary artery pressure [19,20]. This is consistent with our findings showing that the removal of the face mask during ECHO has a positive effect on reducing pulmonary artery pressure in children and adolescents.
The results of our study showed a statistically significant difference in mean HR with and without a mask between the 2 groups. HR decreased slightly after face mask removal, but no significant difference in the mean changes in HR was detected in the case and control groups. Some studies demonstrated that the decrease in HR without masks compared to the time of using the mask stems most likely from the decreased respiratory work required to overcome breathing resistance [19]. Furthermore, in the present study, the difference between the mean of SpO2 in the control group with and without a mask was statistically significant, but it was not significant in the case group. However, the findings of our study suggested no significant difference between the 2 groups following the face mask removal. Our findings are in line with those of Helgeson et al. [20] who showed that wearing a face mask has no discernible impact on arterial SpO2 in patients with moderate-to-severe pulmonary artery pressure. In the same direction, Amput and Wongphon [21] found that wearing cloth masks and surgical masks had no effect on cardiorespiratory parameters, such as HR, blood pressure, and SpO2 at rest and after performing the 6-minute walk test. Moreover, the result of a systematic review by Lott et al. [22] showed that wearing a mask in healthy individuals had no significant impact on the measured physiological parameters, including HR, respiratory rate, and SpO2.
The present study is among the first studies to evaluate the effect of face masks on pulmonary artery pressure through Doppler ECHO in children and adolescents with CHD. The current study's limitation was the use of surgical masks. We expect other mask types to behave similarly to surgical ones. Another limitation was the withdrawal of some patients and their parents in the case group to perform second-stage ECHO during the study.
Further studies with larger samples of children and adults from other locations and ethnicities are recommended for generalisation. In addition, it is recommended that this hypothesis be replicated with a crossover study.
In conclusion, based on results of present study, wearing a face mask increase pulmonary pressure in healthy and CHD in children and adolescent; however, SPO2 and HR remained unchanged. Therefore, we recommend that echocardiographers and physicians remove the mask during ECHO, because mask removal may reduce pulmonary pressure.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: AA, ZSN, MRS; Formal Analysis: ZSN, AA; Investigation: AA, ZSN, MRS; Methodology: AA, ZSN, MRS; Project Administration: AA, MRS; Writing–Original Draft: AA, ZSN, MRS; Writing–Review & Editing:AA, ZSN, MRS
Acknowledgements
The authors would like to thank everyone who contributed to this study, as well as Ms. Mino Diyanat Khah and all participants and their parents who took the time to participate in the study.