Increased serum eosinophilic cationic protein in children with nonspecific chronic cough
Article information
To the editor
Chronic cough in children is common and frequently associated with multiple physician visits [1]. Role of eosinophilic airway inflammation in chronic cough was clearly demonstrated in adults [2], but has not been studied much in children. Recently, it has been reported that eosinophil granule cationic proteins induce cough hypersensitivity and contribute to the pathogenesis of chronic cough [3]. Of those proteins, eosinophil cationic protein (ECP) has been widely studied and is the only available marker for eosinophil activity in clinical practice [3]. Serum ECP has been widely studied as a potential biomarker in asthma [4,5], but it has not been studied yet in chronic cough. We aimed to investigate if evaluation of serum ECP would be useful in management of the children with nonspecific chronic cough.
We enrolled 187 children who presented with chronic cough that lasted more than 4 weeks from March 2020 to August 2022. The subjects were divided by age: group 1 (N=131, school-age, 6–13 years) and group 2 (N=56, preschool-age, <6 years). The patients who were identified to have any specific cause of chronic cough were excluded on enrollment. Exclusion criteria were as follows: (1) premature birth with or without bronchopulmonary dysplasia; (2) any underlying respiratory, cardiac, or immunologic diseases; (3) known asthma diagnosed by their physicians or by bronchial challenge test; (4) good response to antibiotic therapy suggesting protracted bacterial bronchitis or suppurative sinusitis; (5) abnormal findings on chest x-ray and/or high-resolution computed tomography.
Serum ECP was measured using a solid-phase, 2-site chemiluminescent immunometric assay (IMMULITE 2000 XPi immunoassay system, Ehrlangen, Germany). In our study, high ECP was defined as a level of >24 ng/mL (reference value suggested by manufacturer’s manual) and low ECP as a level of ≤24 ng/ mL. Atopic sensitization was defined when the patients had at least one positive reaction to skin prick test or an allergenspecific IgE >0.35 kU/L (Pharmacia Diagnostics, Uppsala, Sweden). Eosinophil count greater than 300/μL was defined as blood eosinophilia [6]. Pulmonary function tests, methacholine challenge tests (MCTs), and measurement of fractional exhaled nitric oxide (FeNO) were conducted in group 1. For statistical analysis, IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA) was used (Supplementary Table 1).
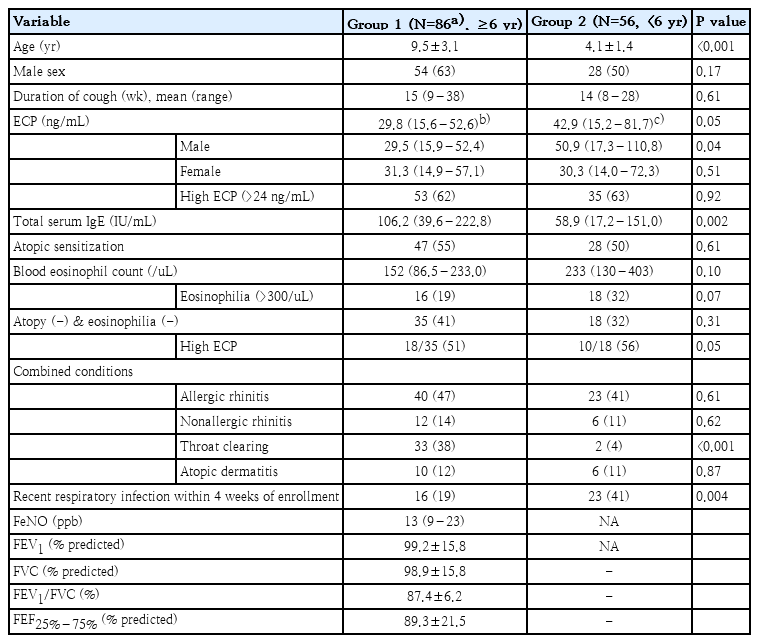
Clinical and laboratory findings of 2 age-groups are presented in Table 1. In group 1, data of 86 patients were finally analyzed because 45 patients with cough variant asthma showing positive MCT (PC20 [provocative concentration of methacholine causing a 20% fall in forced expiratory volume in 1 second] ≤16 mg/mL) were excluded. Serum ECP was significantly increased compared to reference value in both age-groups (P<0.01, respectively), and high ECP was observed in 62% of group 1 and 63% of group 2 showing no age-related difference. Among the patients who had neither atopic sensitization nor eosinophilia, 35 from group 1 and 18 from group 2, 18 (51%) and 10 patients (56%) showed high ECP, respectively. Atopic sensitization and blood eosinophilia are biomarkers that are known to be associated with better response to inhaled corticosteroid [6]. Based on the previous studies which showed the relationship between serum ECP with atopy and blood eosinophils [4,5], our study seems to suggest that evaluation of serum ECP might be helpful in treatment decision in children with chronic cough.
Serum ECP was well correlated with blood eosinophils in both age-groups (Supplementary Fig. 1). In group 1, ECP correlated well with FeNO and FeNO correlated well with eosinophils (Supplementary Figs. 2, 3), and the relationship of ECP with eosinophils and FeNO was still being observed in nonatopic subgroup (Supplementary Fig. 4). These results might be extrapolated to group 2 in whom FeNO measurement was not available. FeNO is a well-known biomarker of eosinophilic airway inflammation in asthma showing a good correlation with eosinophils and ECP in blood, sputum, and bronchoalveolar lavage fluid [7]. KAAACI (Korean Academy of Asthma, Allergy and Clinical Immunology) guidelines recommend FeNO measurement as a diagnostic test to predict steroid-responsive cough in the patients with nonspecific chronic cough [8]. The close relationship among serum ECP, FeNO, and eosinophils shown in school children seems to suggest that serum ECP could help identify the children who might benefit form corticosteroid treatment.
In group 1, significantly higher ECP was observed in those with atopic sensitization versus those without and serum ECP showed a good correlation with serum IgE levels. However, in group 2, serum ECP showed no relationship with atopic status or serum IgE levels. (Fig. 1, Supplementary Fig. 1) A previous study reported that high serum ECP in nonatopic infants with wheezing was predictive of later development of atopic asthma [9]. Taken together, our study suggests that longitudinal evaluation would be needed in preschool children with nonspecific chronic cough and high serum ECP.
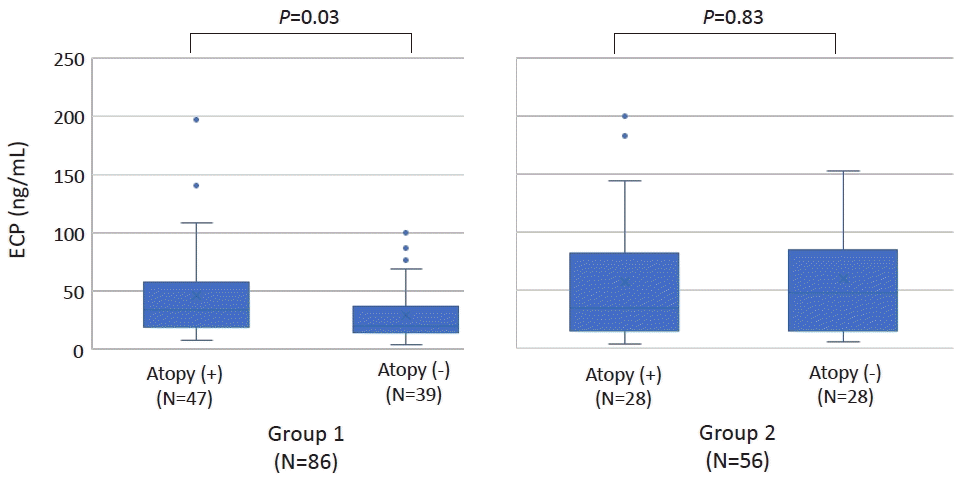
In group 1, the serum eosinophil cationic protein (ECP) level was significantly higher in patients with versus without atopic sensitization (median [interquartile range {IQR}]: 33.9 [19.0–57.4] ng/mL vs. 19.9 [13.7–36.9] ng/mL; P=0.03). However, in group 2, no difference was noted in serum ECP level according to patient atopic status (median [IQR]: 35.2 [15.0–81.7] vs. 48.3 [15.7–84.9] ng/mL; P=0.83). Group 1, school-age, 6–13 years; group 2, preschool-age, <6 years.
The relationship between serum ECP and respiratory infection have been reported showing increased ECP with mycoplasma infection but not with RSV bronchiolitis [10,11]. In our study, PCR tests for respiratory viral and bacterial pathogens were performed in 39 patients with a history of recent respiratory infection. Serum ECP showed no difference among 3 groups: those with positive results, those with negative results, and those with no recent infection and were not tested (Supplementary Fig. 5). Different characteristics of our subjects may be the cause of different results from previous studies, and further studies seem to be needed.
Our study has some limitations. Firstly, we could not evaluate sputum eosinophils and 24 hour-esophageal PH monitoring to define eosinophilic bronchitis and gastro-esophageal reflux cough in our subjects. Secondly, we could not explore the longitudinal change of serum ECP, which seems to be needed in future studies. Despite these limitations, to the best of our knowledge, this was the first study to examine the relationship between serum ECP and nonspecific chronic cough in children.
In conclusion, serum ECP was increased in 62%–63% of children with nonspecific chronic cough, which suggests that eosinophilic airway inflammation might be involved in the mechanism of chronic cough. Serum ECP correlated well with blood eosinophil counts, and in school children, also with FeNO levels. In both age-groups, high ECP was observed in more than half of the patients who had neither atopic sensitization nor eosinophilia. Our study suggests that evaluation of serum ECP might be helpful in management of children with nonspecific chronic cough, especially in children with normal blood eosinophil count or no atopic sensitization.
Supplementary materials
Supplementary Table 1 and Figs. 1-5 can be found via https://doi.org/10.3345/cep.2023.00416.
Statistical analysis
(A, B) In group 1, serum eosinophil cationic protein (ECP) correlated well with serum immunoglobulin E (IgE) level and peripheral blood eosinophil count. (C, D) In group 2, serum ECP showed no relationship with serum IgE level but was highly associated with peripheral blood eosinophil count. Group 1, school-age, 6–13 years; group 2, preschool-age, <6 years.
Serum eosinophil cationic protein (ECP) level highly correlated with fractional exhaled nitric oxide (FeNO) level in group 1. Group 1, school-age, 6–13 years.
Fractional exhaled nitric oxide (FeNO) level highly correlated with blood eosinophil count in group 1. Group 1, school-age, 6–13 years.
(A, B) Serum eosinophil cationic protein (ECP) level highly correlated with blood eosinophil count and fractional exhaled nitric oxide (FeNO) level in nonatopic children, a subgroup of group 1.
There was no difference in serum eosinophil cationic protein (ECP) level among the 3 groups of patients: those who were tested for respiratory pathogen using polymerase chain reaction and had any positive result (N=33; median, 36.5; interquartile range [IQR], 17.8–64.2 ng/mL), those who were tested and had negative results (N=6; median, 32.0; IQR, 19.3–69.2 ng/mL), and those who were not tested as they had no recent respiratory infection within 4 weeks of enrollment (N=103; median, 31.5; IQR, 14.6–65.6 ng/mL). PCR, polymerase chain reaction.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.