Neonatal risk factors associated with attention-deficit/hyperactivity disorder: an umbrella review
Article information
Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder that is being encountered more frequently.
Purpose
In this study, by compiling the evidence from available meta-analyses, an umbrella systematic review was performed of the neonatal risk factors associated with ADHD.
Methods
The PubMed, Scopus, and Web of Science databases were searched for eligible studies. Only systematic reviews were included. Using a random-effects model, 95% prediction intervals were reported for each risk factor. Three studies were ultimately included in the review.
Results
Congenital heart disease, short-duration or incomplete breastfeeding, low birth weight, and 5-minute Apgar scores <7 were significant risk factors for ADHD. However, the quality of the included systematic reviews was low to moderate and the evidence credibility level was suggestive to weak.
Conclusion
The results of this umbrella review proposed that congenital anomalies, short-duration or incomplete breastfeeding, low birth weight, and low Apgar scores were important factors for the manifestation of ADHD symptoms. However, the inclusion of more high-quality studies is needed to validate our results.
Key message
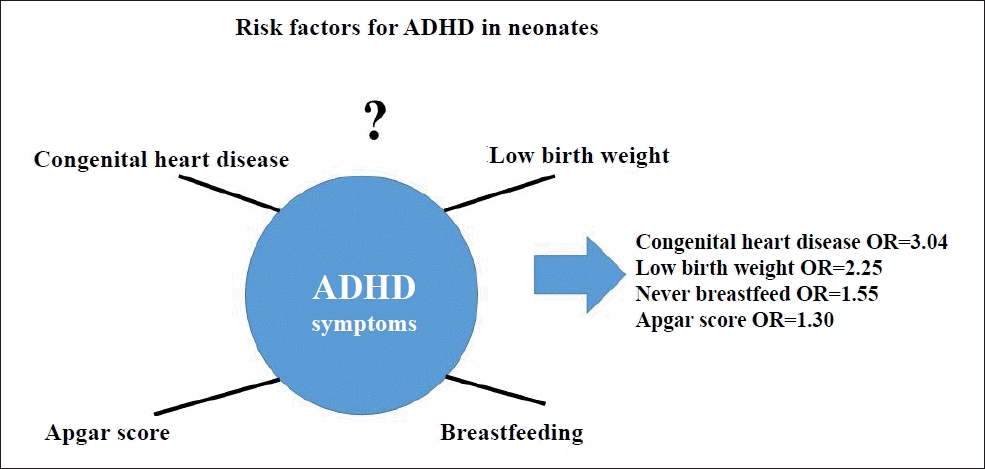
Question: The risk factors for attention deficit hyperactivity disorder (ADHD), such as breastfeeding, congenital heart disease, and low birth weight, in neonates are not well understood.
Finding: This umbrella review obtained significant effect sizes for ADHD for congenital heart disease (odds ratio [OR], 3.04), low birth weight (OR, 2.25), never breastfed (OR, 1.55), and Apgar score (OR, 1.30).
Meaning: Congenital heart disease, low birth weight, lack of breastfeeding, and Apgar scores were significant factors for ADHD.
Graphical abstract. ADHD, attention deficit hyperactivity disorder; OR, odds ratio.
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the common neurodevelopmental disorders characterized by a deficit in attention, hyperactivity, and impulsiveness [1]. The prevalence of ADHD among primary school children was reported to be 11.32% [2]. Even though genetic factors are the most important triggering factor for ADHD, environmental risk factors may contribute to 10%–40% of such a disorder [3]. The environmental factor may interact with genetic factors to trigger the symptoms of ADHD [4,5]. Since most ADHD symptoms manifest in the early life stages, neonatal risk factors have gained special attention regarding the development of ADHD.
Previous studies investigated several risk factors including low birth weight, incomplete breastfeeding, and congenital anomalies [6-9]. Incomplete breastfeeding implies both cases of the absence of breastfeeding or low-duration breastfeeding conditions. Jenabi et al. [8] performed a systematic review for investigating the association between congenital heart disease (CHD) and the risk of ADHD and autism spectrum disorders. The eligible sources were found by a systematic search from major databases including Web of Science, PubMed, and Scopus and the association was found according to odds ratio [OR] and hazard ratio. By adjusting some confounding factors and using a random-effect model, they showed that there was a significant relationship between ADHD and CHD (6 studies; OR, 3.04; 95% confidence interval [CI], 1.58– 4.49; I2=88.1%). Franz et al. [7] using observational studies retrieved from Embase, MEDLINE, PsychINFO, and Cochrane databases investigated the effect of low birth weight on ADHD symptoms. Very low birth weight (VLBW) and extremely low birth weight (ELBW) were defined by weight smaller than 1,500 g and 1,000 g, respectively. They showed a significant association between low birth weight and ADHD (8 studies; OR, 2.25; 95% CI, 1.56–3.26). Furthermore, they showed that for VLBW cases, the association was more significant (4 studies; OR, 4.05; 95% CI, 2.38–6.87). In another study, Tseng et al. [9] investigated the causal relationship between ADHD symptoms and breastfeeding by retrieving relevant studies from PubMed, Embase, ClinicalKey, ScienceDirect, ProQuest, Cochrane Library, and ClinicalTrials.gov databases. The cumulative study using a random-effect model was performed according to Hedge’s g and OR measures for 656 ADHD children and 369 control samples. They reported a significant association between the duration of breastfeeding and ADHD symptoms (8 studies; OR, 1.90; 95% CI, 1.45–2.48; P<0.001). Bitsko et al. [6] searched databases including PubMed, Web of science, and Embase for relevant studies regarding ADHD and pregnancy-related risk factors. The obtained results according to the OR showed a positive association between ADHD and postnatal factors such as Apgar score, neonatal illness, and no breastfeeding (OR range, 1.1–1.6). Even though there are several studies regarding ADHD risk factors, there is a lack of a comprehensive review for compiling the available evidence from existing reviews. The main purpose of the current study was to combine the obtained results of several eligible systematic reviews and meta-analyses regarding the neonatal risk factors for ADHD in an umbrella review framework.
Methods
This umbrella systematic review followed the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.
1. Search strategy
The major databases including PubMed, Web of sciences, and Scopus were used for searching the available eligible studies until April 2022. Only systematic reviews were considered without any constraints on the date of publication or the language. The keywords included: (influencing factors OR related factors OR risk factors) AND (neonate OR neonatal) AND (attention deficit hyperactivity disorder OR ADHD) AND (systematic review OR meta-analysis). The reference lists of eligible studies, systematic reviews, and meta-analyses, were checked carefully for retrieving missed documents. The search procedure, as well as, screening the studies, and assessing the quality of eligible studies were performed by 2 independent authors (EJ and FM) (Supplementary Table 1).
2. Study selection, inclusion, and exclusion criteria
The process of retrieving eligible studies was performed by 2 independent reviewers (EJ and FM). A PICO (participant, intervention, comparison, and outcome) model was used for the current umbrella review. The population was neonatal samples, the intervention was any risk factor, the comparison was performed according to the OR and the outcome was the effect of the risk factor on the manifestation of ADHD symptoms. As inclusion criteria, all systematic reviews and meta-analyses related to the risk factors for ADHD were included. Studies with any type of risk factors, including low birth weight, CHD, and breastfeeding were considered. As exclusion criteria, nonsystematic review studies (including observational studies, letters to the editor, experimental studies, and clinical trials) were excluded. Furthermore, systematic reviews without metaanalysis were excluded (Supplementary Table 2).
3. Data extraction
An electronic form for extracting data from retrieved sources was used. Information regarding the first author’s name, publication year, study design, number of included studies, the number of ADHD samples, effect size, and neonatal risk factors for ADHD was extracted. Furthermore, according to the level of credibility of evidence, each meta-analysis was allocated to one class. When there was an overlap between 2 meta-analyses, the 1 with more included studies was considered. Data extraction was performed by 2 independent authors (EJ and FM) and any disagreement was resolved through discussion.
4. Level of credibility of evidence
For each meta-analysis, the credibility of evidence was estimated. In this regard, the studies were classified into 5 classes: class I (convincing), class II (highly suggestive), class III (suggestive), class IV (weak), and class V (not significant). Criteria for assigning each meta-analysis to one of the classes were P value of random-effect model, P value for the largest study, the total number of ADHD cases, heterogeneity (I2), small-study effect, excess significance bias, prediction interval, and 10% predictability ceiling. The prediction interval indicates the interval for the possible real effect of the risk factor. The credibility ceiling method is a kind of sensitivity analysis to account for the effect of confounding factors and biases (the lack of randomization, measurement errors, or incorrect grouping) in a meta-analysis [10]. The excess significance bias is calculated according to the number of expected studies and the observed number of studies with nominally significant results. The significant level for statistical analyses was adjusted to 0.05.
5. Quality assessment
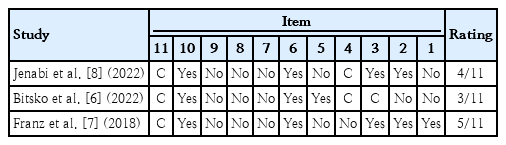
The quality of studies was assessed using the assessment of multiple systematic reviews instrument i.e., a measurement tool to assess systematic reviews (AMSTAR) which was suited for both randomized and non-randomized designs [11]. This is an 11-item tool in which each item should be answered by "yes," "no," "can't answer" or "not applicable." The total score for each study was determined by the number of 'yes' answers. For a total score of 0–3, the quality of meta-analysis was considered low, for scores 4–7, the quality was medium and for scores between 8 and 11, meta-analysis was considered a high-quality study [12].
Results
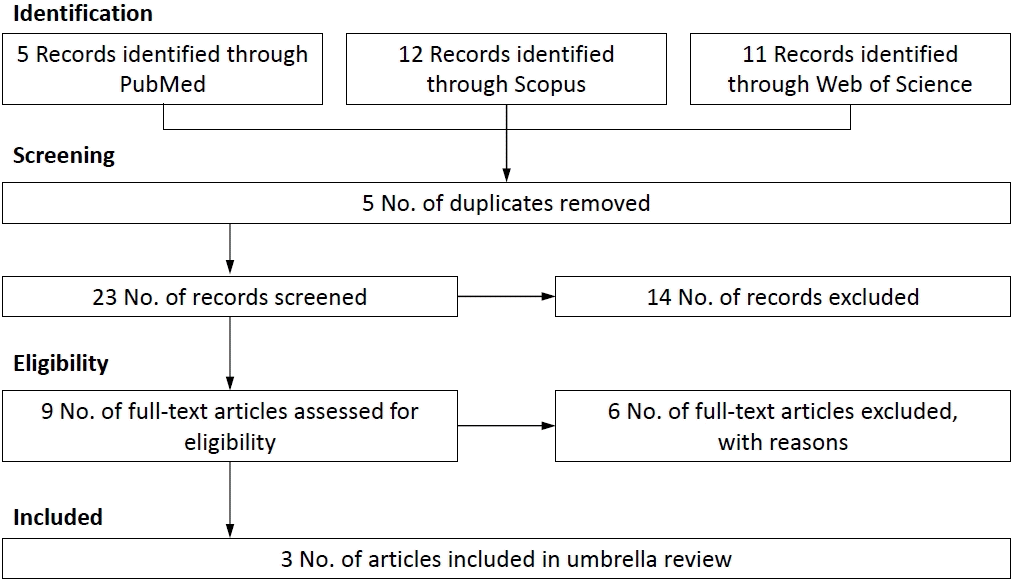
Twenty-eight articles were retrieved from searching 3 main databases until April 12, 2022. Three meta-analysis studies were identified (Fig. 1) which included 6 meta-analyses with 148,455 children with ADHD and 29,927,763 control participants [7-9]. Studies with design of cohort and case-control were included in the present umbrella review (47 original; 22 cohort studies and 25 case-control studies).
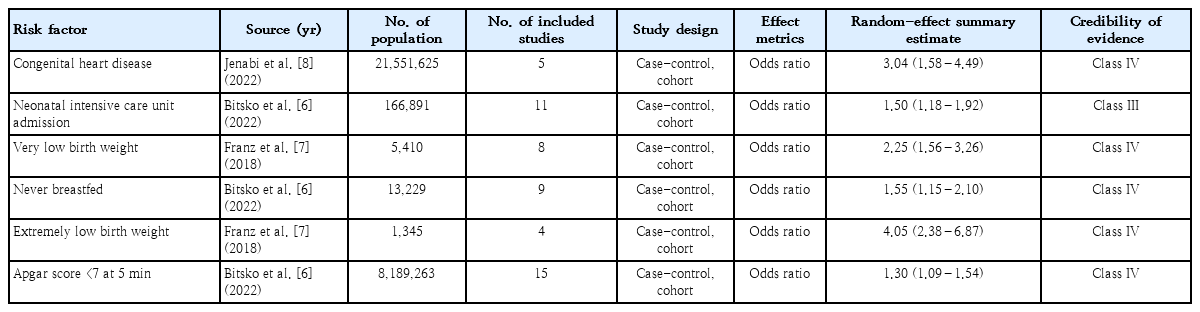
We identified 6 risk factors including CHD, VLBW, ELBW, Apgar score <7 at 5 minutes, never breastfeeding, and neonatal intensive care unit (NICU) admission. CHD is related to structural problems with a baby’s heart and affects the normal functions of the heart. In the current study, all kinds of CHD including mild to severe cases were considered. VLBW is considered for infants with a birth weight lower than 1,500 g, while ELBW is defined for a birth weight lower than 1,000 g. In the present umbrella review, out of 6 included meta-analyses, 4 studies had at least 1,000 cases, 4 presented heterogeneity (I2) <50%, one had small-study effects, and none of the studies had excess significance bias (Table 1). According to the findings of sensitivity analyses, 3 studies were relatively sensitive to unmeasured confounding factors (a bias factor of less than 1.75 in each of the meta-analyses). The other 3 studies (CHD, VLBW, ELBW) were relatively robust to unmeasured confounding (a bias factor of more than 1.90 in each of the meta-analyses).
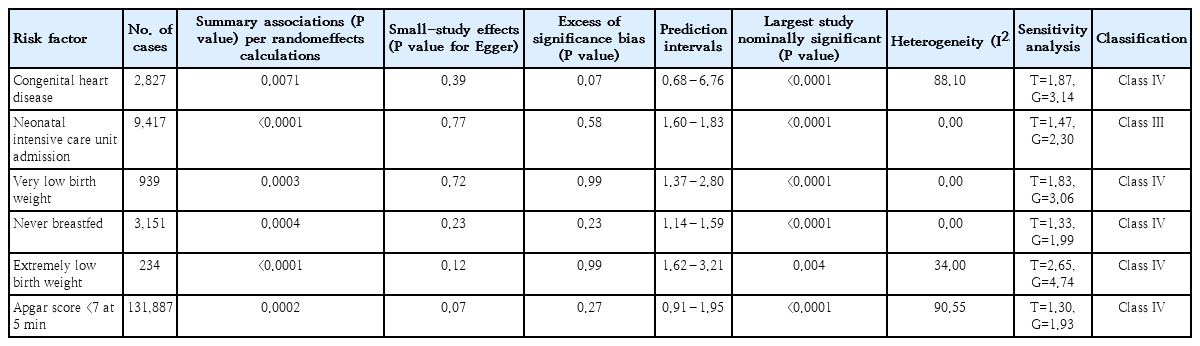
Credibility of evidence of neonatal factors associated with attention deficit hyperactivity disorder
The risk factor of NICU admission (OR, 1.50; 95% CI, 1.18– 1.92) was graded as suggestive evidence (class III). The 5 risk factors of CHD (OR, 3.04; 95% CI, 1.58–4.49), VLBW (OR, 2.25; 95% CI, 1.56–3.26), ELBW (OR, 4.05; 95% CI, 2.38– 6.87), never breastfeed (OR, 1.55; 95% CI, 1.15–2.10), and Apgar score <7 at 5 minutes (OR, 1.30; 95% CI, 1.09–1.54) were graded as weak evidence (class IV) (Table 2). The quality of included meta-analyses was reported based on the AMSTAR checklist (Table 3). According to the AMSTAR checklist, among 3 included meta-analyses, 1 of them was low-quality and the others were medium-quality studies.
Discussion
The evidence level for NICU admission and never breastfed was classified as suggestive (class III), although most of the criteria were met except for the P value (statistical significance; P<0.000001). Since the classification criteria are objective and standard, they are criticized due to arbitrary cutoffs and are prone to misclassification bias [13]. On the other hand, the produced evidence from meta-analyses is highly dependent on the methodology and reporting of the primary and individual studies [14]. Prediction intervals including the null value were observed for CHD and Apgar score <7 at 5 minutes. Moreover, IntHout et al. [15] argued that assumptions for estimating prediction interval can be difficult to establish and these interval may be imprecise in the presence of substantial between-study heterogeneity. Thus, all-or-nothing thinking may be misleading when judging the certainty of evidence from an umbrella review and 2 risk factors of NICU admission and never breastfed can be considered as the higher level of evidence.
The sensitivity analysis showed that the observed associations for NICU admission, never breastfed, and Apgar score <7 at 5 minutes were biased by unmeasured or uncontrolled confounding factors, indicating the observed associations do not imply causation. In other words, the investigators of the individual studies had controlled for only some confounders but not for all of the known or unknown confounders and these unmeasured confounders might be associated with NICU admission, never breastfed, and Apgar score <7 at 5 minutes as well as were associated with ADHD. In this regard, for example in the primary individual studies that aimed to evaluate the association between 2 aforementioned factors, estimating the minimum strength of association that an unmeasured confounder would need to have with both the NICU admission and ADHD can help to understand the true effect of NICU admission on ADHD [16].
The heterogeneity for CHD and Apgar score <7 at 5 minutes were 88.1% and 90.55%, respectively, indicating large betweenstudy heterogeneity. It may be due to the fact that groups of ADHD patients were different across the individual studies in the meta-analyses evaluating the effect of these 2 risk factors and the estimated overall effect may not generalizable to all of the included studies. On the other hand, weak (class IV) evidence level for CHD and Apgar score <7 at 5 minutes may be due to the observed great heterogeneity [17].
The limitations of this umbrella review that should be acknowledged are as follow; according to the AMSTAR checklist, the quality of meta-analyses was low to medium. The quality less than acceptable level was a result of missing information in item 1 (lack of published protocol before conducting metaanalyses), item 5 (lack of reporting inclusion and exclusion criteria in meta-analyses), items 7 and 8 (failure to check the quality of individual studies) and item 9 (weaknesses in the methodology of combining findings of individual studies). All of these potential biases can lead to misleading when interpreting the results from meta-analyses. This review did not cover other important risk factors such as prenatal risk factors. Previous works showed that a combination of prenatal and perinatal risk factors and their interaction plays a pivotal role in the causal pathway of childhood ADHD [18]. Moreover, we acknowledge our review may prone to a degree of selection bias because we only searched 3 databases (PubMed, Web of sciences, and Scopus) and we had only 3 meta-analysis studies including 6 meta-analyses.
In this umbrella review, the current knowledge regarding the risk factors for ADHD in neonatal period was investigated. The results indicated the significant effect of risk factors such as never breastfeeding, VLBW, ELBW, CHD, and NICU admission. The possible reasons for such results may be explained as follows.
1. Congenital heart anomalies
Studies in human and animal samples showed that stresses during early life stages may trigger chronic disease in the future [19]. Furthermore, studies indicated that people with CHD at born had a higher risk of developing neurological deficits [20]. Due to congenital heart defects, the blood flow and consequently the oxygen received to the brain reduces. This hypoxemia condition might significantly affect the brain developmental stages. Hypoxemia also affects highly oxygen-sensitive areas of the brain such as the striate body of the brain and prefrontal cortex which contain important networks for executive attention control [21]. However, it is important to take into account the possible common genetics of ADHD and heart diseases. Patients with 22q11 syndrome show both cardiovascular abnormalities and several psychiatric disorders including ADHD (40% overlap) [22].
2. Low birth weight
ELBW increases the risk for ADHD to 13.8% [23]. Previous studies suggested that ADHD severity was related to poor neurophysiological functioning. The birth weight is an important factor in primary neurophysiological functions and in this way, low birth weight condition affects indirectly ADHD severity and symptoms [24]. For ELBW cases, executive function abilities are degraded compared with normal children [25]. Lower birth weight due to the insufficient supply to the fetus disturbs hypothalamic-pituitary-adrenal axis and increases fetal exposure to glucocorticoid levels. This can affect developmental pathways and trigger psychiatric disorders such as ADHD [26,27].
3. Breastfeeding
Previous studies emphasized the positive role of longer breastfeeding on cognitive development [28]. Such a beneficial effect might be due to the transfer of long-chain polyunsaturated fatty acids to the child through breast milk [29] which enhances the cognitive functions to a large extent [28]. The physical or social interactions between mother and child during breastfeeding and the effects of mother's milk on microbiota are other possible factors that may be responsible for the protective effect of breastfeeding against ADHD symptoms [4].
4. Implications for practice
In this study using an umbrella systematic review framework, the effect of neonatal risk factors on ADHD was investigated. These risk factors were CHDs, breastfeeding, low birth weight, and Apgar score <7 at 5 minutes. The results proposed that such factors were all effective for ADHD manifestation. The small-sample size of the study and the inclusion of low-quality studies were the main obstacles to conclusion and further studies are needed for obtaining a clinically applicable outcome. However, these findings implied that in case of children with CHD, incomplete breastfeeding, low birth weight, or low Apgar score, the symptoms of ADHD should be screened with special attention by the experts and the child's family.
Supplementary materials
Supplementary Tables 1-2 can be found via https://doi.org/10.3345/cep.2022.01396.
Literature search strategy
Studies excluded from meta-analysis with reasons
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was funded by Hamadan University of Medical Sciences, Hamadan, Iran IR.UMSHA.REC.1401.406/No. 140105183629).
Author Contribution
Conceptualization: EJ, SB; Data curation: EJ, FM; Formal analysis: EJ, EA, SF; Funding acquisition: EJ; Methodology:EJ, EA, SF; Project administration: EJ, SB; Visualization: EJ, SF, SB; Writing original draft: EJ, SF, EA; Writing review & editing: SF, FM