Early prophylaxis of cardiomyopathy with beta-blockers and angiotensin receptor blockers in patients with Duchenne muscular dystrophy
Article information
To the Editor
Cardiomyopathy in patients with Duchenne muscular dystrophy (DMD) is often apparent on echocardiography at the age of 15 [1]. Based on the benefits of angiotensin-converting enzyme inhibitors (ACEi) in teen patients with DMD [2], major guidelines and expert working groups have recommended the initiation of treatment with ACEi by the age of 10 years regardless of whether left ventricular (LV) systolic function is normal [3,4].
However, beta-blockers, which are one of the most important heart failure medications, have not been proven beneficial as a prophylactic treatment [5] despite the high incidence of sinus tachycardia and sympathetic prevalence in tween patients with DMD [6]. A recent study reported that beta-blockers did not show any beneficial effect in preserving cardiac function in DMD patients [7]. In the current study, we examined the efficacy of early prophylactic use of beta-blockers and angiotensin II receptor blocker (ARB) by investigating the heart rate (HR)-lowering effect on DMD patients with normal systolic function.
Nineteen patients diagnosed with DMD from May 2013 to July 2021 were reviewed retrospectively. In all of the patients, the diagnosis of DMD was confirmed either by muscle biopsy or DNA analysis. Of these 19 patients, we excluded 2 patients whose 24-hour Holter monitoring results were unavailable. All 17 patients were administered a beta-blocker and either an ARB or an ACEi when their LV systolic functions were still normal on echocardiography.
All patients received follow-up clinical examinations every 3 months. Doses of a beta-blocker and either an ARB or an ACEi were adjusted after physical examinations, which collected vital signs (especially HR and blood pressure) and the patients’ symptoms. Echocardiography was performed in our clinic at least once a year.
In addition to the clinical examinations and echocardiography, 24-hour Holter monitoring was performed to evaluate the patients’ HR and rhythm status annually or biennially. We reviewed the 24-hour Holter monitoring results using the Wilcoxon signed rank test to investigate the effects of treatment with beta-blockers and ARBs. Data are expressed as medians with interquartile ranges (IQRs). Differences were considered statistically significant at P<0.05. This study was approved by the Institutional Review Board (IRB) of Pusan National University Hospital (IRB No. 2112-024-110).
All patients were teenagers. The initial LV ejection fraction (LV-EF) and LV end diastolic dimension were within the normal ranges in all patients. Sixteen patients (84.2%) were administered losartan as an ARB, while 2 patients (10.5 %) were administered Enalapril as an ACEi. All patients received carvedilol as a betablocker. The median follow-up duration was 5.2 years (IQR, 4.0–6.7 years).
There was no mortality during the follow-up years. We detected a decrease in LV-EF below 55% by echocardiography in our subjects at the median age of 15.9 years (IQR, 13.7–18.1 years). There were no serious drug adverse effects such as intolerable hypotension or bradycardia except for syncope in one patient due to orthostatic hypotension.
The 24-hour Holter monitoring results of 7 patients were compared before and 1 year after the treatment (Table 1). The maximal HR was significantly decreased 1 year after the treatment (P=0.028). The average HR and percentage of tachycardia were also decreased but not significantly. HR variability (HRV) indices showed no difference after the treatment. We compared the 24-hour Holter monitoring results of 12 patients treated for 1 year with those of normal, age-matched controls (n=23) (Table 2). Despite treatment with a considerable dose of the betablockers (0.61 mg/kg/day, 0.41–0.91), patients’ average HR and minimal HR were still significantly higher. Patients’ HRV indices (the standard deviation of NN intervals, root-mean-square of successive differences, and NN50 count divided by the total number of all NN intervals), however, were significantly lower (P<0.05).
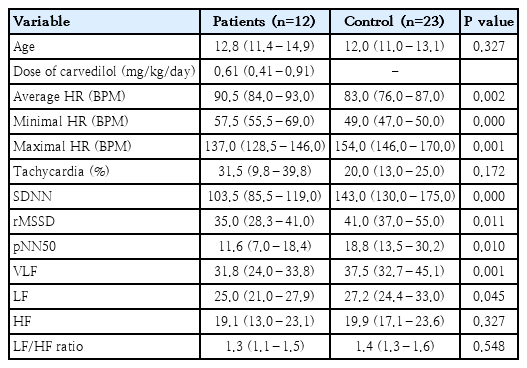
Twenty-four-hour Holter monitoring results of patients at 1-year posttreatment versus normal controls
The current study investigated the HR-lowering effects of early prophylactic administration of a beta-blocker and either an ARB or an ACEi in teenagers with DMD whose LV systolic functions were shown to be normal on echocardiography. Despite 1-year of treatment with beta-blockers, the average HR and minimal HR, but not the maximal HR, of the patients were still higher than those of normal controls. In addition to the HR, diminished HRV, which is strongly associated with myocardial fibrosis [8], was not improved after treatment with beta-blockers. This result suggests that treatment with moderate doses of betablockers and ARBs had limited efficacy in changing the HR and the HRV of patients.
To determine the cause of limited efficacy, it is necessary to consider the mechanism of sinus tachycardia in DMD patients. Sinus tachycardia in teenaged DMD patients is not concurrently correlated with ventricular EF but is, instead, related to autonomic nervous dysfunction defined as sympathetic predominance [9]. In the case of sinus tachycardia with sympathetic predominance, such as inappropriate sinus tachycardia syndrome, beta-blockers are known to have limited efficacy in lowering HR [10]. Other drugs such as ivabradine are reported to be more effective in sinus tachycardia related to sympathetic dominance than betablockers [11].
The median age of the first detection of LV systolic dysfunction (LV-EF<55%) by echocardiography was 15.9 years (IQR, 13.7–18.1 years) in this study. This age is quite similar to that reported by previous study reporting a decrease in LV fractional shortening to below 28% in DMD patients at the age of 15.1±4.2 years [1] without any prophylactic ACEi or beta-blockers. This result is consistent with the findings of a randomized controlled trial reporting that beta-blockers had no beneficial effect as a prophylactic medication [5]. However, beta-blockers and other HR-lowering agents need to be studied further to understand their effects on HR with subsequent reduction in oxygen consumption and stretching stress in DMD patients with normal cardiac systolic function, as sinus tachycardia and sympathetic predominance are related to the cardiac involvement of the parasympathetic branch and precede overt cardiomyopathy [12].
In conclusion, the administration of a moderate dose of a betablocker had limited efficacy in lowering the HR to the normal level in DMD patients with autonomic dysfunction. Hence, other treatment options in addition to beta-blockers or higher doses of beta-blockers need to be examined to lower HR in patients with DMD.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work is supported by Pusan National University School of Medicine and Biomedical Research Institute.
Author contribution
Conceptualization: JYS, ISK, JH; Data curation: HL; Formal analysis: HL; Funding acquisition: HL; Methodology: YBS, JAY; Project administration: JYS; Visualization: HL; Writing - original draft: HL; Writing - review & editing: JYS

