Hypertension in adulthood is programmed during the perinatal period
Article information
Key message
∙ Intrauterine growth restriction (IUGR) and preterm birth can be significant risk factors for the development of adult hypertension.
∙ Several perinatal factors of hypertension are related to IUGR, including renal, vascular, and rapid catch-up growth.
It is well known that intrauterine growth restriction (IUGR) and preterm birth are independent risk factors for the development of adult hypertension [1-3]. In 1988, Barker and Osmond [1] suggested that hypertension created a link between IUGR and adulthood cardiovascular risk and could be established in the womb. A systemic review and meta-analysis based on 10 studies of 1,342 preterm or very low birth weight (VLBW) infants also reported that infants with prematurity or VLBW have higher systolic blood pressure and can be at increased risk for developing hypertension in adulthood [2]. However, IUGR or small for gestational age (SGA) are considered more closely associated with higher rates of hypertension than prematurity alone [3]. According to a review by Altemose and Dionne [3] children born SGA have higher 24 hours, reduced nocturnal, and altered circadian blood pressure rhythmicity.
Although the cause of hypertension is likely multifactorial and not all IUGR cases result in adult hypertension, there are 2 main prenatal factors of hypertension related to IUGR: renal and vascular (Fig. 1) [4]. First, infants with IUGR have a 30%–35% decreased number of nephrons. Brenner et al. [5] reported that a renal abnormality with a reduced number of nephrons, especially due to asymmetrical IUGR, contributed to essential hypertension with salt-sensitivity. A low nephron count leads to the beginning of compensatory mechanisms in the residual nephrons including glomerular hyperfiltration and hypertension; thus, a hyperfiltration injury including proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis develops [6]. Theoretically, hyperfiltration originates from intrarenal renin-angiotensin system (RAS) activation by prenatal programming alters renal sodium handling and advances salt-dependent hypertension [7]. Increased angiotensin II leads to hypertension via multiple mechanisms such as vasoconstriction, aldosterone release, salt and water retention, systemic nerve system tone elevations, inflammation, and fibrosis. Franco et al. also reported that a lower birth weight was associated with higher angiotensin-converting enzyme activity and angiotensin II, and blood pressure, suggesting that perinatal RAS programming continues across the lifespan [8].
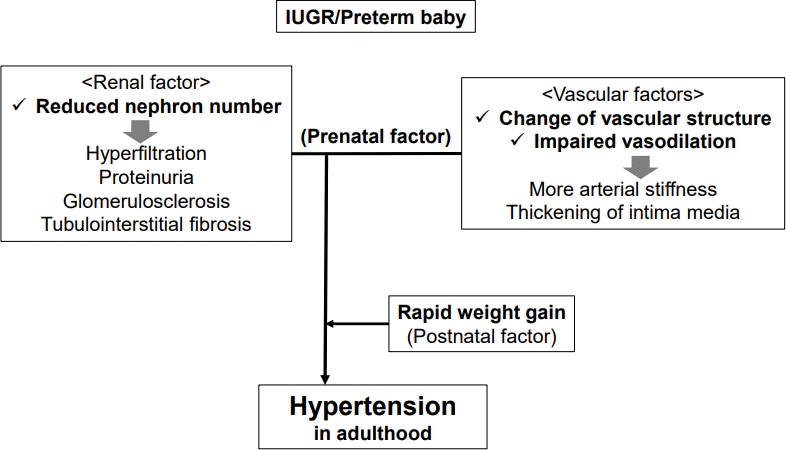
Pathophysiology of perinatal programming between intrauterine growth restriction/preterm birth and hypertension in adulthood. IUGR, intrauterine growth restriction.
Second, preterm infants with IUGR experience changes in vascular structure and impaired vasodilation [9]. These vascular conditions lead to increased arterial stiffness and intimal media thickening, ultimately increasing myocardial workload and leading to hypertension in adulthood. Changes in the vascular structure include impaired vascular elastogenesis and microvascular rarefaction. Elastin, a key element of the extracellular matrix in the media of the vessel wall, generally accumulates in the late prenatal period but decreases quickly after birth. Impaired vascular elastogenesis may reduce the aortic compliance of preterm babies and contribute to the development of hypertension later in life [10]. Decreased density of the microvasculature, such as the arterioles and capillaries, inhibits vascular endothelial growth factor expression and is commonly considered another important factor in the development of hypertension. Impaired vasodilation can be induced by IUGR and preterm birth [9]. Among infants with SGA, substantial impairment of the vascular responses to vasodilators, such as acetylcholine, is considered due to impaired endothelial production of nitric oxide.
An additional postnatal factor of hypertension related to IUGR and preterm birth should not be overlooked. A correlation was reported between rapid weight gain in children with IUGR and hypertension in adulthood. This rapid postnatal weight gain pattern during the first 2 years of life may affect future adiposity and metabolic risk, being consequently linked to hypertension [3,4].
In conclusion, IUGR and preterm birth may be significant risk factors for the development of adult hypertension; therefore, in children with a history of IUGR, attention should be paid to the long-term effects, including those in adulthood, to prevent this predictable complication and establish optimal catch-up growth planning. However, despite evidence of a relationship between IUGR and hypertension in adulthood, confusion persists because this experimental evidence tends to be confined to animal studies and prospective human studies are still lacking. Therefore, additional prospective observational human studies are required to increase our understanding.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported
