Celiac disease in children: Increasing prevalence and changing clinical presentations
Article information
Abstract
Background
Celiac disease (CD) is a chronic autoimmune enteropathy. It results from genetic predisposition and exposure to gluten-containing food. The prevalence and presentation of CD vary among populations.
Purpose
This study aimed to describe the prevalence and clinical characteristics of CD in children in Bahrain.
Methods
We retrospectively reviewed the medical records of children diagnosed with CD in the pediatric department, Salmaniya Medical Complex, Bahrain, in 1988–2018. Their clinical, biochemical, serological, and histopathological findings were documented. Adherence to the recommended gluten-free diet (GFD) was assessed.
Results
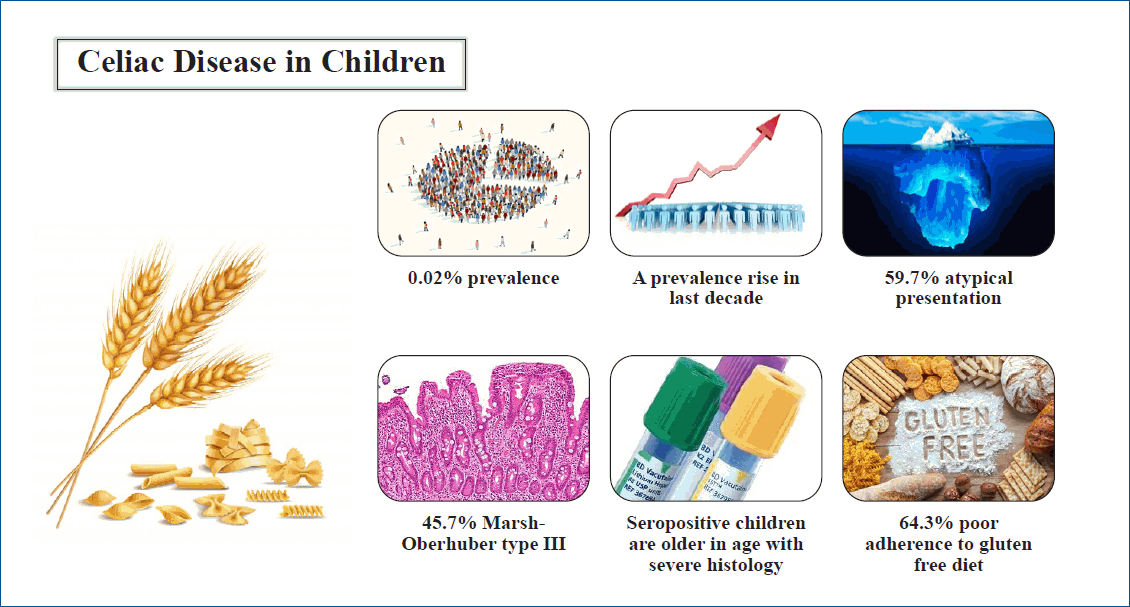
Of 86 patients with CD, 67 were included. The CD prevalence was 0.02%. A significant increase in prevalence in the last decade was observed (P<0.0001). Thirty-eight patients (56.7%) were males. The median (interquartile range) age at presentation was 4.45 (1.5—7.3) years. A family history of CD was positive in 13 out of 43 patients (30.2%). Pallor and failure to thrive were the most common presentations. The most frequent associated disease was iron-deficiency anemia in 23 patients (69.7%). Positive serology was found in 32 of 45 patients (71.1%). Marsh-Oberhuber type III was found in 16 of 35 patients (45.7%). Seropositive patients were significantly older (P=0.025) and had more severe duodenal histology (P=0.002). Adherence to GFD was poor in 27 patients (64.3%).
Conclusion
This study revealed a significant increase in CD prevalence over the last decade. Atypical presentations were frequent. Most patients had poor adherence to GFD.
Key message
Question: What are the prevalence and clinical characteristics of celiac disease (CD) in children in Bahrain?
Finding: We found a significant increase in CD prevalence over the last decade (P=0.0001). A male predominance was noted. Atypical presentations were common. Most patients had poor adherence to a gluten-free diet.
Meaning: CD is an underdiagnosed condition. Atypical symptoms should be considered to prevent missing patients with CD.
Graphical abstract. Prevalence and associated clinical characteristics of celiac disease in children in Bahrain.
Introduction
Celiac disease (CD) is a chronic autoimmune enteropathy [1-5]. It results from genetic predisposition and exposure to gluten-containing food [1,2,4,5]. Individuals carrying human leucocytes antigen (HLA) markers DQ2 or DQ8 are genetically predisposed [4-6]. Gluten is a protein found in wheat, rye, and barley; the main ingredients of bread, pasta, and pastries [2-4,7]. Gluten works as a triggering factor for CD, but the interaction between genetic and environmental factors is still not fully understood [4].
CD prevalence varies widely between populations to be anywhere between 0.5% and 1% [8,9]. The classical symptoms of CD in children are chronic diarrhea, anemia, and failure to thrive (FTT) [10]. However, not all the patients came with the classical presentations. Some may present with food allergy and later on found to have CD [11]. Identifying celiac-specific antibodies by serological tests has allowed us to detect asymptomatic, latent, and atypical cases [6,10]. The diagnosis of CD is confirmed by the presence of histopathological lesions including crypt hyperplasia, an increased intraepithelial lymphocyte (IEL) count (over 25 lymphocytes per 100 enterocytes), with or without villous atrophy within the small intestinal mucosa (Marsh classification type II or III as modified by Oberhuber) [5].
Though CD is a chronic multisystem disease, mucosal damage can be reversed, and antibodies titer drop remarkably if gluten is completely eliminated from the diet [6]. Gluten-free diet (GFD) remains the treatment of choice in CD despite being costly, restraining, and adhering to it is difficult [3]. Recent spread of CD awareness encouraged food industries to produce ranges of harmless substitutes for gluten-containing foods, but the choices are still restricted [3].
CD in children has been evaluated worldwide and in several neighboring countries to Bahrain [1-7,10-21]. To our knowledge, CD in children has not been evaluated in Bahrain. This study aimed to describe the prevalence, clinical, laboratory, endoscopic, and histopathological characteristics of children with CD in Bahrain and to evaluate their adherence to GFD.
Methods
1. Patients and materials
This is a retrospective cross-sectional review of all medical records for patients with CD in the pediatric department, Salmaniya Medical Complex (SMC), Bahrain from January 1988 to November 2018. SMC is the only tertiary hospital in Bahrain and all pediatric patients suspected to have CD are referred to, diagnosed, and managed in this hospital. Accordingly, prevalence and annual incidence of pediatric CD in Bahrain were calculated. Patients over 18 years were excluded. Patients were diagnosed to have CD based on a combination of clinical, serological, and histopathological findings [22]. A combination of at least one positive CD-specific serological test like anti-tissue transglutaminase (anti-tTG) antibodies, antiendomysial antibodies (AEA), or antigliadin antibodies (AGA) along with histologic changes of modified Marsh grade II or more on the small intestinal biopsies is required to diagnose CD [23]. In the absence of serological data, a combination of modified Marsh grade II or more and clinical and/or histological improvements after starting the patient on a GFD are also accepted as a diagnosis criteria [23].
Sociodemographic data were collected including gender, nationality, age at presentation, age at diagnosis, year of presentation, gestational age, delivery type, birth weight, breastfeeding history, time of cereal introduction, and family history of CD. Clinical presentations including pallor, FTT, abdominal distension, short stature, abdominal pain, diarrhea, constipation, and vomiting were collected. Data about the presence of associated diseases were also collected. Laboratory investigations including complete blood count, liver function tests, thyroid function tests, serum iron, ferritin, calcium, phosphorus, and immunoglobulin A (IgA) levels were collected. Anemia was defined as hemoglobin level <11 g/dL in children 6 months to 5 years old, <11.5 g/dL in children 5 to 11 years old, <12 g/dL in children 12 to 13 years old, <13 g/dL in men, and <12 g/dL in nonpregnant women [24]. Iron-deficiency anemia (IDA) was diagnosed based on low serum iron levels with or without low serum ferritin levels [25]. CD-specific antibodies including Anti-tTG, AEA, AGA, and HLA genotyping were recorded. Endoscopic and histological findings of the small intestine biopsies were also collected. Immuno-histochemical staining for cluster of differentiation 3 (CD3) was recorded. Outpatient follow-up period was calculated. The weight and length/height of the last outpatient visit were collected and body mass index (BMI) [weight (kg)/height2 (m)] was calculated. These growth parameters were presented as a standard deviation (SD) from age- and sex-specific reference means, and they were plotted on the relevant World Health Organization (WHO) charts. The WHO standards and references were used to assess the nutritional status. Accordingly, FTT (wasting) and risk of overweight were defined as BMI for age <-2 and >+1 SD, respectively while stunting and severe stunting were defined as length/height-for-age <-2 and <-3 SD, respectively [26,27]. Adherence to GFD was evaluated via reviewing the dietary notes of each outpatient clinic visit.
Based on serological test results, the patients were divided into 2 groups (seropositive and seronegative). The 2 groups were compared according to the age at presentation, gender, clinical presentations, BMI, weight status, height status, presence of associated diseases, hemoglobin levels, Marsh-Oberhuber classifications, and adherence to GFD.
2. Statistical analysis
Patient’s data were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) program. Data were presented as frequency and percentage for categorical variables or mean and SD or median and interquartile range (IQR) for continuous variables. CD incidence and prevalence were calculated after exclusion of non-Bahraini patients. Kruskal-Wallis test was used to compare CD prevalence in the last 3 decades. To compare the 2 serological groups, Pearson chi-square test or Fisher exact test were used for categorical variables. Mann-Whitney U test or Student t tests were used for continuous variables. A 2-sided P value of <0.05 was considered statistically significant.
Results
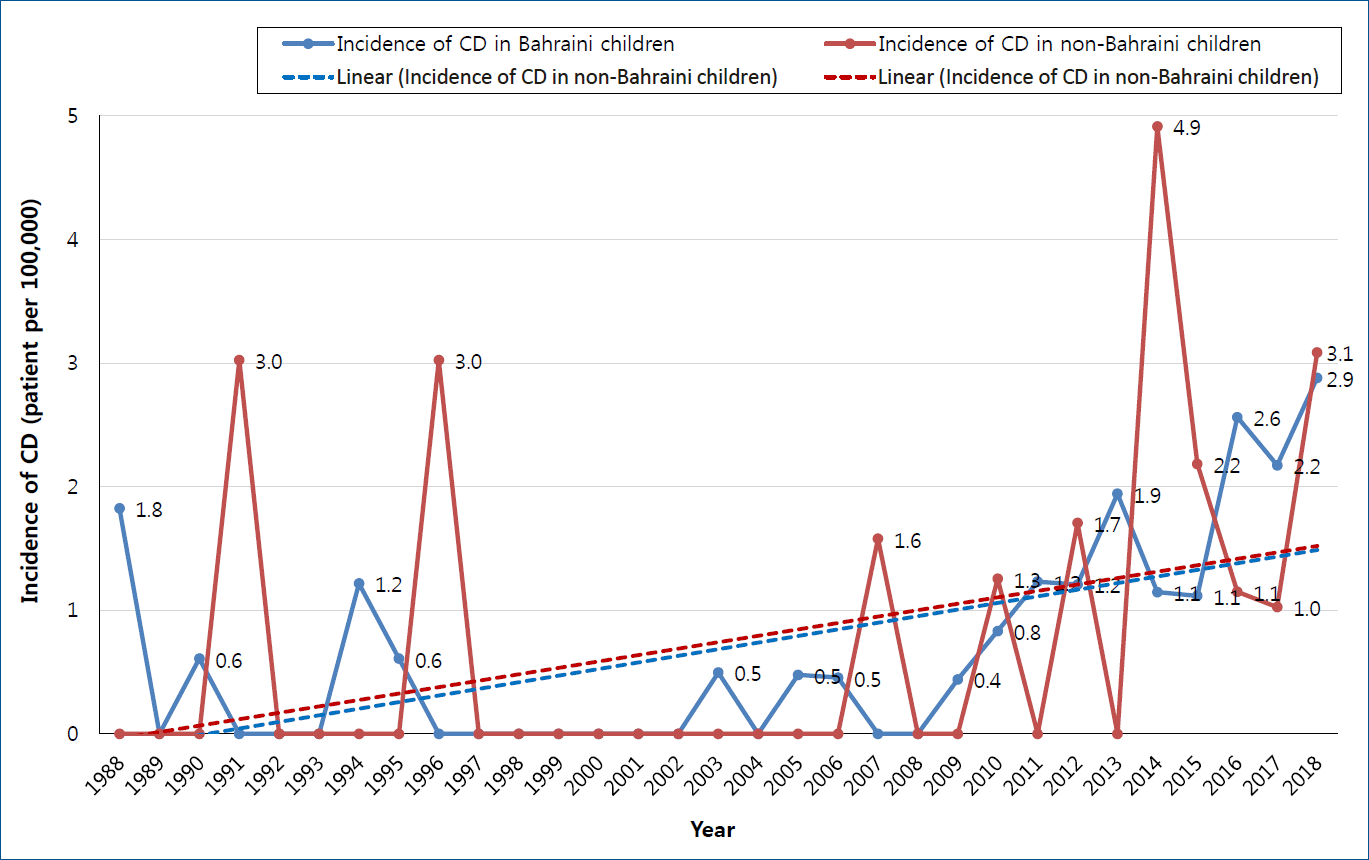
During the study period, 86 patients were diagnosed to have CD. Nineteen patients were excluded (10 patients were more than 18 years of age and 9 patients with unknown age at the time of presentation). The remaining 67 patients were analyzed. According to 2018 Bahrain health statistics, the population of Bahrain is 1,503,091, with 347,886 people aged less than 18 years (277,679 Bahraini and 97,207 non-Bahraini). After exclusion of non-Bahraini patients, the median annual incidence was 0.45 per 100,000 per year (IQR, 0.0–1.2). The annual incidence of CD among Bahraini and non-Bahraini children is shown in Fig. 1. On comparing the numbers of the 48 Bahraini patients in the last 3 decades, 4 patients (8.3%) were diagnosed between 1989 and 1998 (164,470 population at risk), 3 patients (6.3%) between 1999 and 2008 (227,110 population at risk), and 41 patients (85.4%) between 2009 and 2018 (277,679 population at risk). The overall prevalence of CD among Bahraini children is 0.018% (18.36 patients per 100,000). The median (IQR) prevalence of CD in Bahraini children in each decade was 0% (0.0–0.0006), 0% (0.0–0.00046), and 0.0012% (0.001–0.0023), respectively. There was a significant increase in the prevalence of CD over the last 3 decades (P<0.0001).
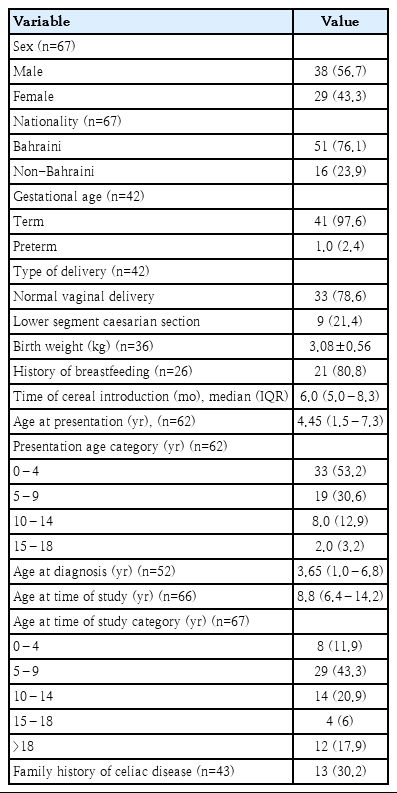
Demographic data are shown in Table 1. Thirty-eight patients (56.7%) were males, and 29 (43.3%) were females. Fifty-one patients (76.1%) were Bahraini, and 16 (23.9%) were non-Bahraini (5 patients were from Pakistan, 3 from Egypt, 2 from Syria, 2 from India, 1 from each Saudi Arabia, Yemen, United Arab Emirates, and Algeria). Median (IQR) age at the time of the study was 8.8 (6.4–14.2) years. Family history of CD was found in 13 out of 43 patients (30.2%); 11 (25.6%) had first degree while 2 had second-degree relatives.
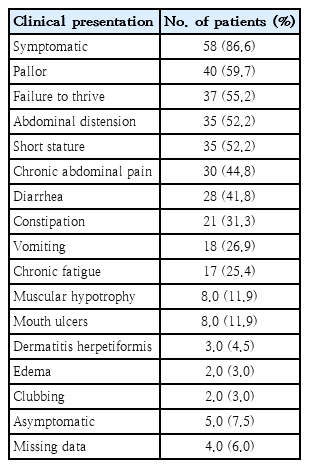
Out of 63 patients (94%) with available detailed clinical history, 58 patients (92.1%) were symptomatic at the time of presentation while 5 (7.9%) were asymptomatic and they were detected via CD screening (3 patients had first degree relative with CD, 1 with autism, and 1 with type 1 diabetes mellitus). The commonest clinical presentations were pallor, FTT, and abdominal distension (Table 2).
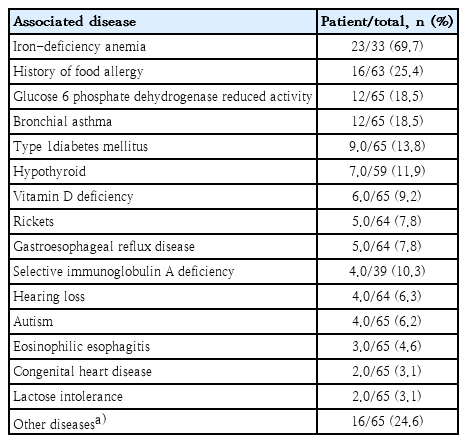
Forty-one out of 65 patients (63.1%) had at least one associated disease (Table 3). The most frequent associated diseases were IDA in 23 patients (69.7%), followed by a history of food allergy in 16 patients (25.4%); 5 of them had multiple food allergies. Dairy product allergy was reported in 6 patients, egg allergy in 4, nut allergy in 3, orange allergy in 2, wheat allergy in 2 while allergy to tomato, seafood, kiwi, lemon, mango, sesame, and antibiotics each in 1 patient. One patient had eosinophilic gastroenteritis and hearing loss.
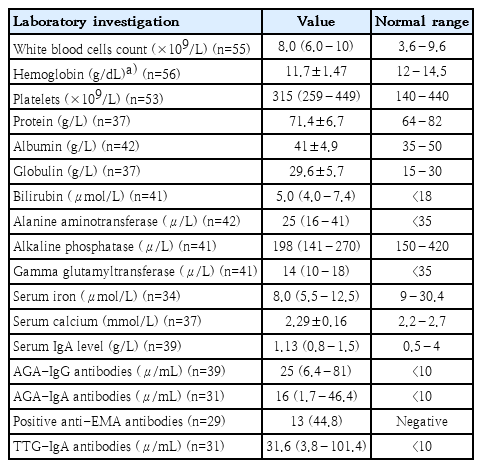
Results of laboratory investigations at the initial presentation are shown in Table 4. Leukocytosis was found in 25.5% (14 of 55 patients). Leukopenia was found in 1 patient (1.8%). Anemia was found in 51.8% (29 of 56 patients) and low serum iron levels in 69.7% (23 of 34 patients). Thrombocytosis was found in 28.3% (15 of 53 patients). Hypoproteinemia was found in 13.9% (5 of 37 patients) while hypoalbuminemia was found in 11.9% (5 of 42 patients) and hyperglobulinemia in 27% (10 of 37 patients). One patient (1.7%) had low serum globulin. Elevated liver enzymes were noted in 30.9% (13 of 42 patients) with high alanine aminotransferases (ALT) in 10 patients (23.8%). Hypocalcemia was found in 37.8% (14 of 37 patients). Serum phosphorus and ferritin were normal in all tested patients; 14 (20.9%) and 35 patients (52.2%); respectively.
Positive CD serology was seen in 32 patients (71.1%) out of 45 (67.2%) with available serological records. Positive AGA-IgG, AGA-IgA, anti-EMA antibodies, and tTG-IgA antibodies were found in 64.1% (25 of 39 patients), 48.4% (15 of 31 patients), 44.8% (13 of 29 patients), and 29% (9 of 31 patients); respectively. All the 8 tested patients for HLA class II types were positive (6 patients for DQ2 alone, 1 for DQ8 alone, and 1 for both DQ2 and DQ8).
All the patients had small intestinal biopsies collected, 63 biopsies (94%) were collected via upper intestinal endoscopy while 4 (6%) via Crosby-Kugler capsules. Out of the 67 patients, 25 (37.3%) had endoscopic data and 35 (52.2%) had histology data available (Table 5). Out of the 25 patients with endoscopic data, 9 (36%), 14 (56%), and 16 patients (64%) had positive findings in esophageal, gastric, and duodenal endoscopies; respectively. Some patients had more than one finding. Findings suggestive of CD (scalloping, shining, and flattening of duodenal mucosa) were noted in 12 patients (48%).
In terms of histological reports, 27 patients (40.3%) had esophageal and gastric biopsy reports available while 35 patients (52.3%) had available duodenal biopsy reports. Out of 35 patients with duodenal reports, 18 (51.4%) had histological findings suggestive of CD (Marsh II or III). Marsh III was seen in 16 patients (45.7%) with CD histological findings. IEL were detected in 51.4% (18 of 35). Immuno-histochemical staining for CD3 showed increase in IEL in the all 3 tested patients. Out of 31 patients with available antral biopsy cultures, 2 patients (6.5%) were positive for helicobacter pylori.
The median (IQR) follow-up period was 9.3 years (6.4–21). Three patients (4.5%) died, 1 passed away secondary to an associated myeloproliferative disease while the other 2 patients from Saudi Arabia and United Arab Emirates died out of Bahrain with unclear cause. According to the last outpatient visit anthropometric data, weight was available for 47 patients (70.2%) and height for 44 (56.7%). Thirty-seven patients (78.7%) had normal weight, 8 patients (17%) were wasted, and 2 (4.3%) were at risk of overweight. In terms of height, 38 (86.4%) had normal height, 5 (11.4%) were stunted, and 1 (2.3%) was severely stunted. Adherence to GFD was strict in 15 (45.7%) and poor in 27 (64.3%) out of 42 patients (62.7%) with available data.
Comparison between children with CD according to the serological tests results is shown in Table 6. Patients with positive CD serology were significantly older in age (P=0.025) and had more severe duodenal histology based on Marsh-Oberhuber classification (P=0.002). There was no significant difference found between seropositive and seronegative patients in regard to sex, clinical presentations, BMI, weight status, height status, presence of associated diseases, hemoglobin levels, or adherence to GFD.
Discussion
The present study showed an overall prevalence of CD in children from Bahrain of 0.02% with a significant increase in the prevalence during the last 3 decades. However, CD prevalence in the present study is lower than that reported globally where prevalence of CD is ranging between 0.7% and 1.4% [6,7,9,28]. Globally, CD prevalence has also increased overtime from 0.6% (1991–2000) to 0.8% (2001–2016) [28]. Moreover, a study from United Kingdom reported that the prevalence of CD among children older than 2 years had tripled over the 20 years between 1993 and 2012 [13]. Similar to our study, most of the patients with CD from Kuwait were diagnosed in the second half of the study period (between 1998 and 2010) [14].
In this study, the estimated median annual incidence of CD in children is 0.45 per 100,000 people per year (IQR, 0.0–1.2). This incidence is also low when compared to studies from Italy and United Kingdom which reported incidence of 11.9 and 11.8 per 100,000 person-years; respectively [12,13]. This variation in the prevalence and incidence of CD between different countries can be attributed to vague presentations and delayed diagnosis in one hand; and to increased number of screened high-risk groups and/or increased awareness among physicians about CD on the other hand [6,14,29,30].
CD tends to manifest more in children aged 0–4 years [12]. The current study showed that 33 patients (53.2%) presented before the age of 4 years followed by a decline in the number reaching only 2 patients (3.2%) between the age of 15 and 18 years. This decrease in incidence with age is also shown by studies from India and Italy [10,12]. Despite the fact that CD is considered an infantile disease, diagnosis in later childhood is not uncommon [7,10]. There is an upward shift of age at diagnosis of the disease due to the change in the clinical presentations from malabsorption syndrome to a milder form [2,29].
The current study showed more male patients (n=38, 56.7%) affected with CD compared to 29 females (43.3%). This finding is in contrary to what has been consistent in several studies of different populations where a higher incidence in females was noticed [1-3,5,6,10-15,17-19,28]. The present study also showed a positive family history of CD in 13 (30.2%). Likewise, studies from Saudi Arabia and Finland revealed positive family history in 29.2% and 26.4%; respectively [3,15]. However, Kuwait study reported affected first degree relatives in only 9% [14].
CD can present with either typical (symptomatic) or atypical (asymptomatic or screened) presentations [9]. In the current study, 58 patients (86.6%) were symptomatic while 5 (7.5%) were asymptomatic. Similarly, studies from Saudi Arabia and Italy showed a majority of symptomatic patients of 92% and 60.4%, respectively [3,12]. Younger age at presentation is considered a consistent finding of typical disease presentation while children with atypical disease presenting later in life [2,11]. This could be owed to the fact that younger patients present with classical symptoms such as diarrhea, FTT, abdominal distension, and anemia which are easy to recognize [2,7,10,11,14]. On the other hand, older children happened to complain of vague symptoms and often missing any gastrointestinal (GI) symptoms [11].
CD has a broad spectrum of clinical presentations [14]. Moreover, changes in the CD clinical presentation have become obvious since 80s [30]. Extraintestinal symptoms such as anemia, short stature, bone, hepatic and neurological issues have become more recognized [30]. CD in children may be considered a systemic disease as extraintestinal manifestations dominate CD presentations of more than half of patients [30]. In the present study, the commonest presentations were pallor, FTT, abdominal distention, short stature, and chronic abdominal pain. However, a prospective study from India showed that diarrhea, FTT, and abdominal distension are the major symptoms of the disease while abdominal pain, vomiting, and constipation are relatively less common [10]. In contrary, a study from Saudi Arabia showed that the commonest presentation was chronic abdominal pain followed by poor weight gain [3]. Yet, recurrent mild abdominal pain is a common complaint in patients who were diagnosed upon screening [14].
Pallor is considered as one of the atypical presentations of CD [14]. However, it was the commonest presentation in this study and was found in 40 patients (59.7%). Similarly, pallor was found in 15 out 25 symptomatic patients (60%) from Kuwait [14]. Moreover, pallor was seen in 100% of 134 patients from India [10]. Conversely, studies from Saudi Arabia and Turkey reported anemia as a clinical presentation in only 12.4% and 5.1% patients; respectively [1,3].
While FTT was the most frequent symptom of patients from Turkey (81.4%) and Kuwait (72%), the current study demonstrated FTT in 37 patients (55.2%), being the second commonest clinical presentation [2,14]. Similarly, Saudi and Indian studies reported FTT in 54% and 52.2%, respectively, as a second most common presentation [3,10]. A retrospective study from Finland looked into the etiology of decreased growth in 530 patients with CD found that an earlier age of onset, severe clinical, serological, and histological finding are associated with disturbed growth at CD diagnosis [15]. Despite that FTT is a common complain, Kuwaiti study documented patients who managed to reach overweight (14%) and obesity (6%) among patients with CD [14].
Short stature was seen in 32 patients (52.2%) in the present study. Short stature was documented in 81.4% Turkish patients [2]. Yet, another study from Turkey found only 16.6% to be short [1]. Short stature was also reported in 22%, 20.9%, and 17% of children with CD from Saudi Arabia, India, and Kuwait; respectively [3,10,14]. Like our study, a case-control study from Germany did not show a significant difference in height between CD seropositive and seronegative children despite that seropositive children tended to be shorter [6].
CD is associated with many immune and some nonimmune related diseases. A variety of endocrine abnormalities have been reported to accompany or precede CD diagnosis [1,2]. Patients with insulin-dependent diabetes mellitus, thyroid disorders, Addison diseases, pernicious anemia, autoimmune thrombocytopenia, sarcoidosis, alopecia, and cardiomyopathies have higher risk of CD than normal population [1,5]. In the present study, associated conditions were found in 63.1% (41 of 65). History of food allergy was found in 25.4% (16 of 63). Likewise, Finnish study also reported concomitant chronic diseases in 61% (315 of 517) and food allergy in 24.1% (125 of 518) [15]. Yet, an American study found only 0.97% (4 of 411) of patients to have associated food allergies [11].
Type 1 diabetes mellitus commonly precedes CD diagnosis (10%–25% of cases) and it is more common in patients with CD compared to general population [1,2]. In this study, type 1 diabetes mellitus was seen in 13.8% (9 of 65) which is similar to the American study (12.8%, 10 of 78) [11]. Patients from Saudi Arabia and Oman had higher percentage of associated diabetes of 26.5% (30 of 113) and 22.2% (2 of 9), respectively [3,7]. However, studies from Turkey and Finland showed lower percentages of 6.4% (9 of 140) and 7.3% (41 of 516), respectively [2,15].
Nonspecific laboratory changes were documented in patients with CD [2]. In the present study, low hemoglobin was noted in 51.8%. Anemia was the most dominant laboratory feature in patients with CD from Turkey (56.4%), India (100%), and Kuwait [2,10,14]. Yet, only 37.4% of patients from Finland and 33.3% of patients from Mexico were anemic [15,18]. More specifically, IDA was seen in 23 patients (69.7%) in our study. This is double than the prevalence of IDA in children aged 3 years from Bahrain (30%) [31]. Similar to our study, studies from India and Turkey also reported IDA in 60.4% and 45.7% respectively while a study from Mexico showed lower percentage (25%) [2,10,18]. In this study, IDA was diagnosed based mainly on low serum iron levels with or without low serum ferritin levels. However, all the patients tested for serum ferritin levels had normal values. This finding is expected because serum ferritin is an acute-phase reactant and can be raised in patients with infections, chronic diseases, or chronic inflammation [25].
Elevated liver enzymes were noticed in 13 patients (30.9%) in the current study with high ALT in 10 patients (23.8%). CD-associated liver involvement has been noted by multiple studies; ranging between 34.8% to 38.8% [2,10,14,15]. Liver enzyme derangements are more frequently seen in symptomatic patients with CD and patients with Down syndrome, nephrotic syndrome and juvenile rheumatoid arthritis [10,14,15].
Autoantibody testing made a significant breakthrough in diagnosing both symptomatic and asymptomatic CD [6]. More than 80% of patients with CD from Italy were diagnosed by both positive serology and histology [12]. In this study, the overall autoantibody tests were positive in 32 (71.1%) out of 45 patients (67.2%) with available data (AGA IgG in 64.1%, AGA IgA in 48.4%, AEA IgA in 44.8%, and tTG IgA in 29%). This is lower than values reported by other studies [2,14,10,19]. These differences might be explained by the presence of high percentage of IgA deficient patients (10.3%) in our study compared to other studies where IgA deficiency was found in only in the range between 0.3% and 6.3% [2,4,7,10,12,18].
HLA markers remain the strongest risk factor for development of CD [4]. In the current study, 8 patients were tested for HLA class II types and all of them were positive (100%). Likewise, studies from Turkey and Mexico showed an overall positive HLA class II types in 97.4% and 73.3% of patients, respectively [1,18].
In the present study, endoscopic data were found in 25 patients (37.3%), 16 of them (64%) had abnormal duodenal findings. In a study from Poland, variable disease extensions have been documented by endoscopy [5]. Both continuous and interrupted focal intestinal lesions have been found [5]. In untreated patients, continuous lesions extending from the proximal part of duodenum to jejunum are more likely [5]. A study from Turkey revealed that 2 out of 14 patients (14%) had normal endoscopic findings and one had unclassified sprue [1].
Traditionally, intestinal biopsy and histological assessment have been mandatory criterion standard of CD diagnosis [1,18]. Histological findings have higher sensitivity and specificity than HLA-DQ2/DQ8 and tTG-IgA positivity combined [1]. Although duodenal biopsy is very important in CD diagnosis, symptomatic cases with very high tTGA levels, positive AEA, and the disease-related HLA types can be excluded from being biopsied [1,20]. In the current study, 35 patients (52.2%) had duodenal histological reports available. Eighteen patients (51.4%) had histological findings suggestive of CD (Marsh II or III). The majority of these patients (88.9%, 16 of 18) had Marsh III. Other studies also had a majority of Marsh III [4,10,12,14]. Histological findings vary between patients [10]. In the current study, Marsh IIIc was the commonest type found in 22.8% (8 of 35) compared to type IIIa in 17.1% (6 of 35) and type IIIb in 5.7% (2/35). This is close to percentage reported from Italy and United States of America where Marsh IIIc was found in 37.5% (42 of 112) and 32% (132 of 411), respectively [4,11]. However, Marsh IIIb was the commonest in patients from India, Finland, and Iraq [10,15,19].
In the present study, Marsh 0 was found in 28.6%, Marsh I in 20%, and Marsh II in 5.7%. A study from Italy reported Marsh 0 in 13.4%, Marsh I in 9.8%, and Marsh II 4.5% [4]. In the current study, Marsh 0 was higher than the Italian study which can be explained by the older age at presentation in their patients [4]. It is more common to observe advanced histopathological stages in older children [11]. Another explanation is that IEL in the present study were detected on hematoxylin and eosin-stained samples in 51.4% (18 of 35). IEL is considered a vital clue to diagnosis [20]. Unfortunately, these cells could be missed due to lack of contrast especially when counts are low [20]. A more sensitive method is immune-histochemical staining for CD3 which can improve the diagnosis accuracy. This is shown in a prospective study on 159 patients where a difference of 12.6% was found between the 2 methods [20]. In the present study, immuno-histochemical staining for CD3 was tested in 3 patients and showed increase in IEL in all of them.
Up to this date, the most efficient treatment for CD is strict adherence to GFD [3,11]. Yet, 64.3% of patients in the current study showed poor adherence to GFD. Patients who were followed up 1 year after starting GFD reported better general health status and less gastrointestinal symptoms, but adherence declined by 29% and even further down with time [3,6]. The latter could be attributed to high cost and unavailability of trustworthy gluten-free products [3]. In addition, as patients grew older more temptations develop and the parents lose control over diet especially since patients become more adventurous with their diet options and more influenced by peer pressure [3,6].
In this study, patients with positive CD serology were significantly older in age (P=0.025) compared to seronegative patients. This might be attributed to the longer exposure to gluten-containing diet. However, a study from India on 66 children with CD up to 18 years of age did not show a significant correlation between serology and advancing age [32].
In the current study, more severe duodenal histology was noted in seropositive patients based on Marsh-Oberhuber classification (P=0.002). This finding was also reported by other studies in which the severity of small intestinal damage on histology was correlated with tTG IgA levels [10,32,33]. A study from Iraq showed a significant reduction in the levels of CD-specific antibodies along with histological remission after GFD which also support our finding [19].
Similar to any other retrospective studies, this study was limited by missing some relevant data. Moreover, low sample size was another limitation that can be explained by the underestimation and underdiagnoses of the disease that is a well-documented issue in other studies [6,14]. In addition, despite that SMC is the only tertiary care center in Bahrain, this study remains a single-center study and its’ results cannot be completely generalized to the whole population. However, this study is the first study from Bahrain focusing on the clinical and pathological characteristics of patients with CD and it can form a valuable source of information for any future studies tackling the same group of patients.
In conclusion, although CD is an underdiagnosed disease, this study revealed a significant rise in CD prevalence in the last decade. Clinical findings are widely variable with atypical presentations are likely to be faced. Pallor, FTT, and abdominal distension were the most frequent presentations. IDA and allergic diseases were the commonest associated diseases. Marsh IIIc is the commonest positive histological finding which indicates more severe disease. Most patients had poor adherence with GFD. Further studies are required to focus on the degree of public and medical staff awareness about CD; and to find out the reasons behind poor adherence to GFD in this group of patients.
Notes
Ethical statement
This study was conducted in accordance with the principles of Helsinki Declaration and it was ethically approved by the secondary care medical research subcommittee, Salmaniya Medical Complex, Ministry of Health, Bahrain (IRB number: 04-04-2016).
No potential conflict of interest relevant to this article was reported.