Particulate matter and childhood allergic diseases
Article information
Abstract
Particulate matter (PM) is a ubiquitous air pollutant that is a growing public health concern. Previous studies have suggested that PM is associated with asthma development and exacerbation of asthma symptoms. Although several studies have suggested increased risks of atopic dermatitis, allergic rhinitis, and allergic sensitization in relation to PM exposure, the evidence remains inconsistent. The plausible mechanisms underlying these effects are related to oxidative stress, enhancement of sensitization to allergens, inflammatory and immunological responses, and epigenetics. This review discusses the effect of PM on childhood allergic diseases, along with plausible mechanisms. Further studies are required to understand the role of PM exposure on childhood allergic diseases, to reduce these diseases in children.
Introduction
Air pollution has been suggested as an important environmental risk factor for allergic diseases. Among air pollutants, particulate matter (PM) is a growing public health concern. PM is a complex and heterogeneous mixture of tiny solid or liquid particles suspended in a gas [1] that is generated from fossil fuel combustion by road transport and power plants; industrial processes, such as the production of metals, cement, lime, and chemicals; construction work; cigarette smoking; and wood stove burning [1]. PM contains acids, organic chemicals, hydrocarbons, metals, and biological material (e.g., endotoxins, allergens, and pollens) [1-3]. PM is categorized according to its aerodynamic diameter: PM10 (smaller than 10 μm), coarse PM (ranging from 2.5 to 10 μm), PM2.5 (smaller than 2.5 μm), and ultrafine PM (smaller than 0.1 μm) [2] Several studies have investigated the relationship between allergic diseases and PM. In this review, we discuss the effects of PM on childhood allergic diseases and the possible mechanisms.
Association of PM and childhood allergic diseases in epidemiologic studies
1. Asthma
1) Asthma incidence and prevalence (Table 1)
Several birth cohort studies have reported a positive association between increased PM and asthma incidence [4-7]. A birth cohort study in the United States (US) demonstrated that increased prenatal exposure to PM2.5 during 16–25 weeks of gestation is associated with asthma onset by the age of 6 years in boys [8]. In addition, PM2.5 exposure at the birth address increases the risk of new-onset asthma (odds ratio [OR], 3.1; 95% confidence interval [CI], 1.3–7.4) and bronchial hyperresponsiveness at the age of 7 years in children with a family history of asthma [9]. In a systematic review, prenatal exposure to PM10 was found to be significantly related to asthma development (OR, 1.08; 95% CI, 1.05–1.12) but not exposure to PM2.5 (OR, 1.02; 95% CI, 0.97–1.03) [10]. Another meta-analysis also showed an association between increased PM2.5 and PM10 exposure and asthma development (for 1 μg/m3 PM2.5, overall risk estimate, 1.03; 95% CI, 1.01–1.05 and for 2 μg/m3 PM10, overall risk estimate 1.05; 95% CI, 1.02–1.08) [11].
Other cohort studies have not found a significant association between PM and asthma incidence. For example, a Canadian cohort study found that PM10 exposure during pregnancy increased asthma onset in young children (age <6 years: adjusted OR [aOR], 1.12; 95% CI, 1.05–1.19), whereas it did not affect school-aged children (age ≥6 years: aOR, 1.09; 95% CI, 0.96–1.24) [12]. Moreover, PM2.5 and PM10 exposure during the first year of life and the first 3 years of life were not associated with childhood asthma onset in Latino and African American populations [13]. In a cohort of 2,497 children in kindergarten and first grade in the US, new-onset asthma was not associated with PM2.5 and PM10 during 3 years of follow-up [14]. In a population-based birth cohort study, an increased risk of asthma incidence (up to age 14–16 years) was associated with increased exposure to PM2.5 absorbance at the birth address, but not at the current address [15]. There were also no significant associations between PM2.5 absorbance and asthma prevalence in that study [15]. A British birth cohort study showed that asthma prevalence from birth to 11 years of age was not associated with PM10 exposure during the first year of life and throughout the lifetime [16]. Further, a meta-analysis of cross-sectional studies conducted in 12 European countries showed no association between PM10 and asthma prevalence [17]. The European Study of Cohorts for Air Pollution Effects project found no significant association between PM exposure and asthma prevalence in 5 European birth cohorts [18]. The reasons for these differing results among epidemiologic studies may arise from differences in exposure and outcome measurement, study population, and study design.
2) Asthma exacerbation (Table 2)
Several studies have reported an association between increased PM levels and the exacerbation of asthma-related symptoms, with a high degree of consistency [19-25]. In a multicity study in Korea, an interquartile range (IQR) increase in PM10 (30.7 μg/m3) resulted in an increase of 2.4% in asthma-related hospitalization among children aged 0–14 years [19]. PM10 was also found to increase the risk of asthma-related hospitalization (aOR, 1.02; 95% CI, 1.01–1.04) in a nested case–control study of children aged 3–18 years in France [20]. An observational time-series analysis in the US showed a 26% increase in intensive care unit admissions and a 19% increase in general hospitalizations for each 12-μg/m3 increase in PM2.5 [21]. The Inner-City Asthma Study in the US revealed that higher PM2.5 is associated with significantly lower forced expiratory volume in 1 second and peak expiratory flow (PEF) in children with moderateto-severe asthma [22]. A meta-analysis revealed a significant association between PM2.5 levels and moderate or severe exacerbation of asthma (OR, 1.022; 95% CI, 1.000–1.045) [23]. Another meta-analysis showed that a significant relationship exists between exposure to PM10 (aOR, 1.013; 95% CI, 1.008–1.018) and PM2.5 (aOR, 1.025; 95% CI, 1.013–1.037),with increased emergency room visits and hospital admissions [24]. Another meta-analysis found that an increase of 10 μg/m3 in PM10 increases asthma symptoms by 2.8% and decreases PEF by 0.082 L/min [25].
2. Allergic rhinitis (Table 3)
The role of PM in childhood allergic rhinitis (AR) is not well established. Although some epidemiologic studies suggest increased risks of AR in relation to PM exposure, the evidence remains inconsistent. Different AR definitions, exposure assessments, and study designs might explain these inconsistent results. In a crosssectional study of 2,661 kindergarten children in Taiwan, lifetime PM2.5 exposure increased the risk of lifetime AR (OR, 1.54; 95% CI, 1.03–2.32) [26]. Annual mean exposure to PM10 (OR, 1.16; 95% CI, 1.04–1.28) and PM2.5 (OR, 1.38; 95% CI, 1.08–1.78) was associated with current AR in children aged 8–9 years, in a cross-sectional study in England [27]. Another cross-sectional study in France showed that the 3-year average concentration of PM10 (OR, 1.20; 95% CI, 1.01–1.44) was associated with increased lifetime AR in children aged 9–11 years [28]. A cohort study of 2,598 children aged 3–6 years in China showed that PM10 exposure during the first year of life increased lifetime AR (OR, 1.54; 95% CI, 1.07–2.21) [29]. A pooled meta-analysis of 6 birth cohorts showed a significant association between PM2.5 exposure at the birth address and AR at the age of 7–8 years (OR, 1.37 per 5 μg/m3 PM2.5) [30].
Some studies have reported no association between PM and AR. Lifetime AR was not associated with PM10 in a study of 6,730 Chinese children aged 3–7 years [31]. AR prevalence was also found to not be associated with PM10 in a nationwide cross-sectional study among Taiwanese children aged 6–15 years [32]. No association between PM10 exposure during pregnancy and AR incidence in children aged 3–6 years was found in a prospective cohort study in China [33]. A meta-analysis of cross-sectional studies determined that there was no association between PM10 and AR diagnosis (OR, 1.20; 95% CI, 0.99–1.46) [17]. A population-based birth cohort study reported that no associations were identified between PM at the birth or current addresses and AR incidence or prevalence up to the age of 14–16 years [15].
3. Atopic dermatitis (Table 4)
Longitudinal studies examining the effects of PM on exacerbation of atopic dermatitis (AD) symptoms reported similar findings. In a prospective study of 22 Korean children diagnosed with AD aged 16–85 months, for every 1-μg/m3 increase in PM10, AD symptoms were increased by 0.44% on the following day [34]. A 10-μg/m3 increase in PM10 increased AD symptoms by 3.2% in children aged <5 years in a panel study in Korea [35]. Another study in Korea showed that an IQR increase in PM10 (24.0 μg/m3) and PM2.5 (12.7 μg/m3) increased AD symptoms (OR, 1.063; 95% CI, 1.006–1.123 and OR, 1.078; 95% CI, 1.018–1.141, respectively) in children aged <6 years [36], and another longitudinal study among 41 children aged 8–12 years in Korea found that pruritus severity was significantly associated with ultrafine PM [37].
There is little evidence on the association between PM exposure and AD prevalence and incidence, and the available results are inconsistent. Further research is needed to investigate the impact of PM on AD prevalence and incidence. One study showed that the 3-year average concentration of PM10 was associated with both lifetime AD (OR, 1.13; 95% CI, 1.01–1.24) and 1-year history of AD (OR, 1.15; 95% CI, 1.03–1.33) in a cross-sectional study among 4,907 French children aged 9–11 years [28]. A prospective Korean birth cohort study found an association of PM10 exposure in the first trimester with AD development at the age of 6 months (OR, 1.219; 95% CI, 1.023–1.452, per 10 μg/m3 PM10) [38].
A cross-sectional study in the US found a positive association between mean annual PM2.5 and moderate-to-severe eczema (OR, 1.070; 95% CI, 1.013–1.130) in children aged 0–17 years.39) However, PM2.5 (OR, 0.993; 95% CI, 0.989–0.998) and PM10 (OR, 0.847; 95% CI, 0.739–0.971) have been found to be inversely associated with eczema prevalence in another study [39]. A nationwide survey of middle-school students in Taiwan also showed a negative association between mean annual PM10 exposure and eczema among girls (OR, 0.79; 95% CI, 0.70–0.89) [40]. The inverse association between outdoor PM and AD might be explained by protective climate factors and a difference in the measured and actual levels of PM exposure [39,40]. A cross-sectional study on 2,661 kindergarten children in Taiwan showed no association between PM2.5 or PM10 and lifetime AD [26]. This is consistent with German and Dutch birth cohort studies that found no association between long-term PM2.5 exposure and AD at the age of 6 or 8 years [4,41]. Additionally, PM10 during the prenatal period and 3 months after birth was not associated with AD at the age of 6 months in a Taiwan birth cohort study [42]. Finally, a prospective cohort study in China showed that PM10 exposure during pregnancy was not associated with AD incidence in children aged 3–6 years [43].
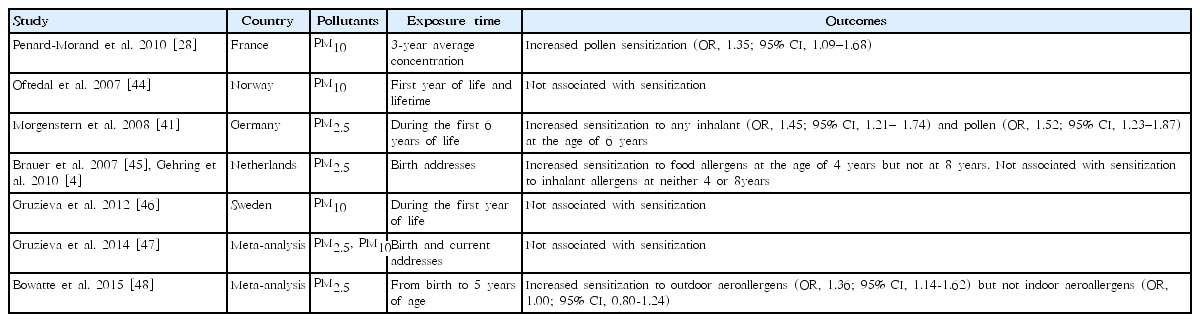
4. Allergic sensitization (Table 5)
The association between PM and allergic sensitization is not well established due to the inconsistent results across studies. Pollen sensitization was significantly associated with 3-year average concentration of PM10 (OR, 1.35; 95% CI, 1.09–1.68) in a cross-sectional study among French children aged 9–11 years [28]. A population-based study including children aged 9–10 years in Oslo did not show a significant association between PM10 exposure in the first year of life or lifetime PM10 exposure and sensitization to any allergen [44]. However, PM2.5 during the first 6 years of life increased sensitization to any inhalant (OR, 1.45; 95% CI, 1.21–1.74) and pollen (OR, 1.52; 95% CI, 1.23–1.87) at the age of 6 years in a German birth cohort study [41]. In a Dutch birth cohort study, PM2.5 exposure at the birth address was associated with sensitization to food allergens at the age of 4 years but not at 8 years; associations between sensitization and inhalant allergens were not significant at either 4 or 8 years [4,45]. A Swedish birth cohort study also found no clear associations between PM10 exposure during the first year of life and overall sensitization at the age of 4 and 8 years [46]. A meta-analysis of 5 European birth cohorts showed no clear associations between PM2.5 and PM10 exposure and allergic sensitization in children up to 10 years of age [47]. Finally, another meta-analysis of birth cohort studies reported significant associations between early childhood PM2.5 exposure and sensitization to outdoor aeroallergens but not indoor aeroallergens [48].
Mechanisms
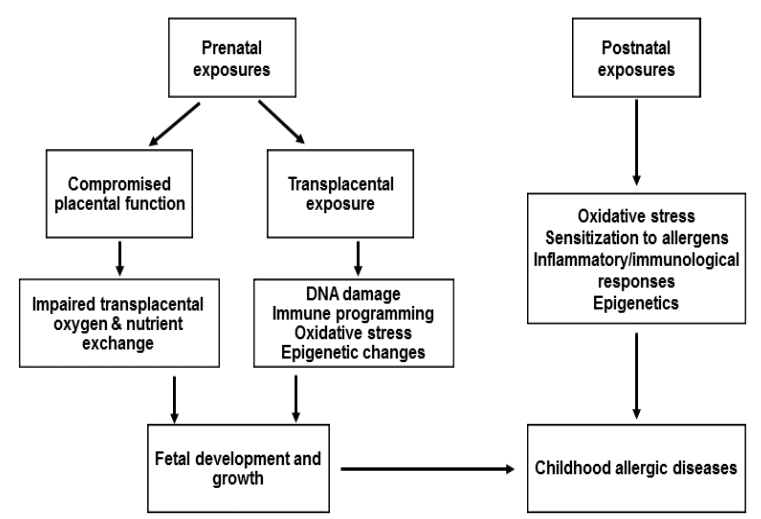
The mechanisms by which PM induces effects on childhood allergic diseases remain unclear. Oxidative stress and damage, enhancement of sensitization to allergens, inflammatory pathways and immunological responses, and epigenetics may have roles in childhood allergic diseases caused by PM. The plausible mechanisms by which PM affects childhood allergic diseases are summarized in Fig. 1.
1. Oxidative stress and damage
PM forms reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide, and hydroxyl radicals [2]. These ROS may directly damage proteins, lipids, and DNA [49]. For example, ROS modifies surfactant protein, which enhances lipid peroxidation, inflammation, and oxidative damage within the lung. Lipid peroxidation, or the oxidative degeneration of lipids, can damage cell membranes and can ultimately lead to cell death. In addition, an end-product of lipid peroxidation, 4-hydroxy-2-nonenal, can lead to airway remodeling by increasing fibronectin production and activating epidermal growth factor receptor. PM-induced DNA damage alters gene and protein expression as well as increases cell death. In addition, oxidative stress activates mitogen-activated protein kinase signaling, activator protein 1, and nuclear factor-kB, which is responsible for the expression or activation of proinflammatory cytokines (e.g., interleukin [IL]-4, IL-6, IL-8, and tumor necrosis factor [TNF]-α), chemokines, and adhesion molecules [3,50-53].
2. Enhancement of sensitization to allergens
PM can increase sensitization to allergens in several ways. Deposition of allergens in the airways increases owing to allergen carriage by PM. PM induces oxidative stress, leading to an increase in epithelial permeability and subsequent recruitment of cells involved in the allergic response. PM also modifies antigenic protein expression, leading to an exaggerated allergenic response. Furthermore, PM increases sensitization to allergens by acting as an adjuvant, thereby preventing antigens from dispersing, activating antigen-presenting cells, and stimulating the division of type 2 helper T-cells [3,51].
3. Inflammatory pathways and immunological responses
PM induces the release of inflammatory cytokines (e.g., IL-6, IL-8, granulocyte-macrophage colony-stimulating factor, and TNF-α) from immune and bronchial epithelial cells [52]. PM are recognized by pathogen recognition receptors (PRRs) on antigen-presenting cells and Toll-like receptors and nucleotide-binding oligomerization domain-like receptor families. Once PM is detected by PRRs, different signaling pathways are activated to upregulate the expression of inflammatory genes (e.g., IFN-α, IFN-β, and TNF-α). PM also promotes Th2 responses [52]. PM upregulates the expression of the dendritic cell (DC) maturation marker CD86, which is involved in priming Th2 responses. PM also activates DCs and basophils in vivo to produce Th2-associated cytokines IL-4 and IL-5 [52].
4. Epigenetics
Epigenetic changes may play a role in regulating the expression of genes related to allergic diseases. Exposure to ambient polycyclic aromatic hydrocarbons during pregnancy is associated with increased DNA methylation of the acyl-coenzyme A synthetase long-chain family member 3 promoter in cord blood DNA and is related to increased asthma risk in children [54]. Increased air pollution is also associated with hypermethylation of the FOXP3 gene in the peripheral blood of children with asthma, impairing regulator T-cell function and increasing asthma morbidity [55].
5. Prenatal exposure to PM
PM can act directly through translocation via the placenta or indirectly by compromising placental function [56]. Transplacental exposure to PM-induced oxidative stress and by-products of PM metabolism damage DNA, alter gene function, and subsequently affect fetal development [56]. Prenatal PM exposure may also influence immune programming. One study showed that increased PM2.5 levels during 14 days prior to birth was associated with decreased levels of T lymphocytes and increased B lymphocytes in cord blood [57]. Higher prenatal PM10 exposure decreased the expression of cytokines IL-10 and IL-1β and increased the percentage of CD4+ CD25+T-cells in cord blood [58,59]. Higher exposures to PM2.5 during midpregnancy were found associated with a higher prevalence of elevated cord IgE [60]. Increased levels of ROS, placental inflammation, maternal blood viscosity, and endothelial dysfunction could be associated with inadequate placental perfusion, which would subsequently impair transplacental oxygen and nutrient exchange and consequently affect fetal development [56,61].
Conclusions
There is considerable evidence that PM is an important risk factor in childhood allergic diseases. Oxidative stress, enhanced sensitization to allergens, inflammatory and immunological responses, and epigenetics are considered possible mechanisms. PM exposure during the prenatal period may also affect childhood allergic disease by way of direct transplacental exposure or possibly by compromising placental function. Further studies are needed to identify the mechanisms by which PM causes allergic diseases and to identify factors that increase the susceptibility of children to PM and allergic diseases, such as genetic polymorphisms and critical exposure windows. Effective new preventive and therapeutic approaches are also needed. Precise exposure assessments, as well as biomarkers of exposure and health effects, are required to accurately evaluate the association between PM and childhood allergic diseases. Strict monitoring and avoidance of PM exposure, as well as new clinical and therapeutic strategies, could helpto prevent childhood allergic diseases.
Notes
No potential conflict of interest relevant to this article was reported.