Association between vitamin D level at birth and respiratory morbidities in very-low-birth-weight infants
Article information
Abstract
Purpose
This study aimed to evaluate vitamin D status at birth in very-low-birth-weight infants (VLBWIs: <1,500 g) and to determine the association between vitamin D level and respiratory morbidity.
Methods
A retrospective study was conducted at Soonchunhyang University Bucheon Hospital between November 2013 and November 2017. We collected blood samples and data on respiratory morbidity from 230 VLBWIs on the first day of life. Patients who were transferred to other hospitals (n=19), died before 36 weeks of gestational age (n=18), or whose blood samples were not collected immediately after birth (n=5) were excluded. Finally, 188 patients were enrolled. VLBWIs with different vitamin D levels were compared with respect to demographic features, maternal diseases, respiratory morbidities, and other neonatal diseases.
Results
The mean serum vitamin D level, as measured by 25-hydroxyvitamin D (25(OH)D), was 13.4± 9.3 ng/mL. The incidence of vitamin D deficiency (<20 ng/mL) was 79.8%, and 44.1% of preterm infants had severe vitamin D deficiency (<10 ng/mL). Logistic analysis shows that a low serum 25(OH)D level (<20 ng/mL) was a risk factor for respiratory distress syndrome (odds ratio [OR], 4.32; P=0.010) and bronchopulmonary dysplasia (OR, 4.11; P=0.035).
Conclusion
The results showed that 79.8% of preterm infants in this study had vitamin D deficiency at birth. Low vitamin D status was associated with respiratory morbidity, but the exact mechanism was unknown. Additional studies on the association between vitamin D level and neonatal morbidity are required.
Introduction
Vitamin D is a fat-soluble vitamin that plays a major role in calcium and phosphorus homeostasis and bone metabolism in the body. It is involved in innate and acquired immune responses and autoimmune responses, and it inhibits cancer cell proliferation, regulates cardiovascular function, and regulates hormones such as insulin [1].
The fetus itself does not produce vitamin D, but receives vitamin D from the mother. A verylow-birth-weight infant (VLBWI; birth weight <1,500 g) has a short gestational age, resulting in reduced vitamin D received from the mother, poor nutritional support, and poor exposure to sunlight during neonatal intensive care unit (NICU) hospitalization.
Therefore, vitamin D deficiency is common and increases the likelihood of neonatal morbidities and death [2-4]. The relationship between vitamin D deficiency and preterm infant diseases such as sepsis [5,6], necrotizing enterocolitis (NEC) [7], respiratory distress syndrome (RDS) [8], and bronchopulmonary dysplasia (BPD) [9,10] is under investigation.
RDS is diagnosed in 50% of VLBWIs and is a common cause of premature infant death [11]. BPD accounts for 23% of VLBWIs and 52% of preterm infants 501 to 750 g [12].
According to Nguyen et al. [13], vitamin D plays a role in the interactions between mesenchymal cells and alveolar epithelial cells and is involved in the maturation of the fetal lung. Type II alveolar cells express vitamin D receptor and are involved in the synthesis and secretion of surfactants in response to vitamin D. The role of vitamin D in pulmonary development and maturation and postnatal respiratory diseases has been researched as a new field of study. However, to best of our knowledge, no study has reported the association between vitamin D level and preterm morbidities in VLBWIs in Korea. In this study, we reviewed the clinical outcomes at NICUs and the incidence of respiratory diseases as it relates to vitamin D levels immediately after birth.
Materials and methods
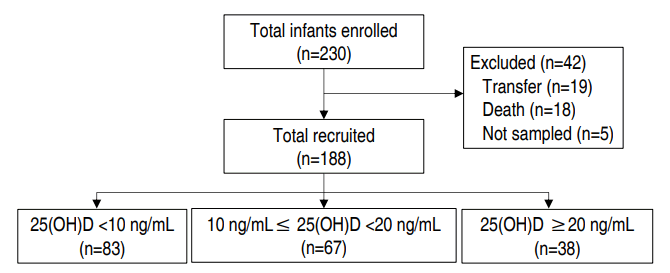
We retrospectively reviewed the medical records of VLBWIs who were admitted to the NICU between November 2013 and November 2017 at Soonchunhyang University Bucheon Hospital (latitude 37.5ºN). Infants who were transferred to another hospital (n=19; mean duration of hospitalization was 36.1±28.9 days; range, 3–96 days) or whose blood was not sampled (n=5) were excluded. Infants who died before 36 weeks of gestational age (n=18) were also excluded to satisfy the diagnostic criteria of BPD. Fig. 1 shows a flowchart of the study course.
Finally, 188 patients were enrolled in the study, and vitamin D was measured within 24 hours of birth. The clinical parameters were birth weight, gestational age, sex, mode of delivery, Apgar score (1- and 5-min), twin birth, birth season, hospitalization period, death, and oxygen therapy method and duration.
The seasons were defined as spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). Premature infant diseases include RDS, BPD, pneumothorax, pulmonary hemorrhage, sepsis, NEC, retinopathy of prematurity (ROP), patent ductus arteriosus (PDA), periventricular leukomalacia (PVL), and intraventricular hemorrhage (IVH). Maternal illnesses that were shown to be associated with vitamin D deficiency, such as gestational diabetes mellitus (DM), premature rupture of membranes (PROM), and preeclampsia, were estimated [14,15].
The Institutional Review Board of Soonchunhyang University Bucheon Hospital approved the medical record review for this study and exemption from patient consent (approval number: 2017-08-014).
1. Definitions of morbidities and serum measurements of vitamin D
RDS was diagnosed based on clinical symptoms, chest X-ray, and blood gas analysis. BPD was diagnosed on the basis of radiographic findings and the need for and methods of O2 therapy after 28 days of age.
We decided to divide O2 therapy into invasive methods and noninvasive methods. Ventilator application was defined as an invasive method regardless of the ventilator mode. On the other hand, nasal prong, high flow nasal cannula, noninvasive positive pressure ventilation, and nasal continuous positive airway pressure were considered as noninvasive O2 therapy. We documented the respiratory severity score (RSS) in intubated preterm infants (RSS=FiO2× mean airway pressure) on days 1 and 21.
Sepsis was diagnosed when blood cultures were positive and systemic symptoms appeared.
NEC was diagnosed according to the modified Bell et al.’s staging [16] criteria based upon the severity of systemic, intestinal, and radiographic findings. PDA was diagnosed based on clinical symptoms and echocardiography. ROP was diagnosed by an ophthalmologist through retinal examination according to the schedule of the American Academy of Ophthalmology [17].
IVH and PVL were diagnosed using brain ultrasound and brain magnetic resonance imaging (MRI). The initial brain ultrasound was performed within 72 hours of birth, and rescreening was performed at intervals of 1 to 4 weeks. In cases of an abnormal brain ultrasound or neurological symptoms, a brain MRI was performed prior to discharge.
Samples were collected from a peripheral vein within 24 hours of life, and vitamin D was measured in 25-hydroxyvitamin D (25(OH)D), the main circulatory form. The 25(OH)D levels were measured by the ultra-performance liquid chromatography-tandem mass spectrometry and chemiluminescence immunoassay method. Alkaline phosphatase (ALP) was measured by a colorimetric method.
According to the Holick et al., [18] vitamin D deficiency is defined as a 25(OH)D level below 20 ng/mL (50 nmol/L), and vitamin D insufficiency is defined as a 25(OH)D level of 21–29 ng/mL (52.5–72.5 nmol/L). In this study, subjects were classified into 3 categories: group 1, severe vitamin D deficiency (25(OH)D <10 ng/mL); group 2, deficiency (10–20 ng/mL); group 3, insufficiency (20–30 ng/mL) plus sufficiency (≥30 ng/mL). We analyzed whether the frequencies of variables differed between groups.
Vitamin D was initially administered via M.V.H. (Whanin Pharm Co., Ltd., Seoul, Korea). It contains vitamin D2, and the dose was 100 IU/kg. After the start of enteral feeding, vitamin D was supplemented with breast milk, preterm formula, breast milk fortifier, and vitamin D drops. We used Sunny D Drops (GMP Laboratories of America, Inc., Anaheim, CA, USA), which contain vitamin D3. There were no established standards for supplementation of vitamin D in the early period of this study. After February 2016, we administered 400 IU for vitamin D sufficiency, 1,200 IU for severe deficiency, and 400 to 800 IU for values in between; however, these were not absolute standards.
2. Statistical analysis
Data were analyzed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). The qualitative data were presented as numbers and percentages while quantitative data were presented as means and standard deviations. Comparisons of qualitative data between groups were done using the chi-square test and/or Fisher exact test. Comparisons of quantitative data between groups were done using analysis of variance. Logistic regression analysis was used to assess the relationship between 25(OH)D levels and premature infant diseases. A P value of less than 0.05 was considered significant.
Results
This study enrolled 188 VLBWIs. Patients with vitamin D deficiency accounted for 79.8% (n=150) of the study population. The number of neonates with severe vitamin D deficiency was 83 (44.1%), and 38 (20.2%) had vitamin D insufficiency or sufficiency.
The patients demographic features are presented in Table 1. Overall, the neonates’ mean gestational age was 28.4±3.0 weeks of gestation, and their mean birth weight was 1,104.7±298.1 g. Male neonates accounted for 52.7% of the study population, and no significant differences in weight were found between groups according to sex. Serum ALP concentrations were significantly higher in patients with severe vitamin D deficiency.
The average 25(OH)D level in boys (12.5±8.5 ng/mL) was lower than that in girls (14.3±10.1 ng/mL), but the P value was not significant (P=0.2). One hundred thirty-two infants (70.2%) were singleton births, and 56 (29.8%) were twin births. The birth weight of group 1 was 1,045.2±293.8 g, while that of group 3 was 1,245.7± 267.1 g. There was no difference between groups with respect to birth season (P=0.075). However, when we compared vitamin D levels by birth season, the mean value of vitamin D was 10.4±7.7 ng/mL in spring, 15.7±9.5 ng/mL in summer, 16.2±10.9 ng/mL in autumn, and 12.0±8.6 ng/mL in winter, which were statistically significant (P= 0.007). There were significant differences in birth weight (P<0.001), Apgar score at 1 minute (P=0.007), and maternal age (P=0.031).
The mean maternal age was 33.2±4.2 years. The frequency of maternal disease (PROM, DM, preeclampsia) was not significantly different depending on the vitamin D level.
The clinical outcomes are shown in Table 2. The average duration of hospitalization was 65.1±51.3 days for group 1, 49.8±28.1 days for group 2, and 42.3±18.1 days for group 3. This difference was statistically significant (P=0.005). The total number of deaths was 6, with no significant difference between groups. In the oxygen therapy period, the total O2 usage period, the mechanical ventilation period, and the noninvasive O2 usage period were all significantly higher in group 1 than in the other groups (P<0.001).
RSS on days 1 and 21 were both higher in the group with lower vitamin D levels, and this difference was statistically significant. Pearson correlation demonstrated a negative correlation between vitamin D levels and RSS on day 1 (P<0.001, r=-0.38), as seen in Fig. 2.
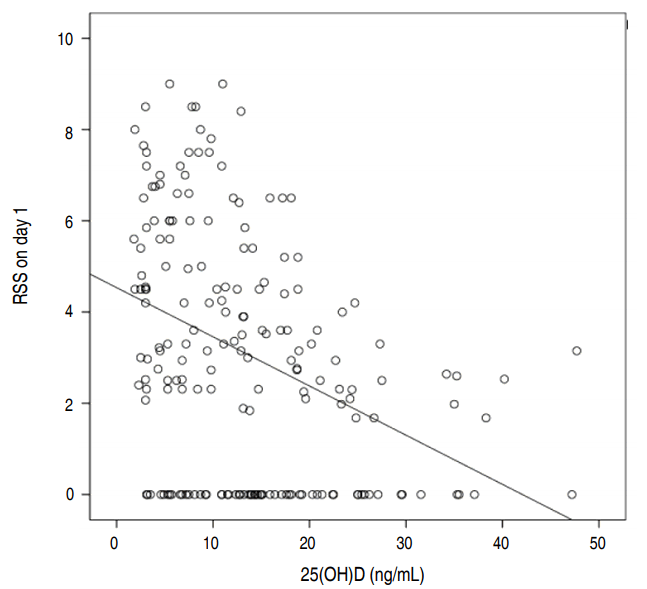
Pearson correlation between 25(OH)D and respiratory severity score (RSS) on day 1. 25(OH)D, 25-hydroxyvitamin D.
To evaluate the association between neonatal morbidities and vitamin D levels, we compared the rate of RDS among the 3 groups. There were 72 cases (86.7%) of RDS in group 1, 45 cases (67.2%) in group 2, and 16 cases (42.1%) in group 3, a statistically significant difference (P<0.001). Similarly, there was a higher rate of BPD in the lowest vitamin D group, and this difference was statistically significant (P<0.001). RDS and BPD were more frequent in group 1, although the percentage of surfactant usage was significantly higher in group 1.
In neonates with severe vitamin D deficiency, ROP was diagnosed more often (P=0.001). There was no significant difference in the incidence of the following morbidities: pneumothorax, pulmonary hemorrhage, PDA, NEC, sepsis, PVL, and IVH (grades III and IV).
In Table 3, logistic regression analysis shows that vitamin D deficiency at birth was a risk factor for neonatal morbidities (RDS: odds ratio [OR], 4.32; P=0.010; BPD: OR, 4.11; P=0.035; and ROP: OR, 5.49; P=0.032).
Discussion
Recently, several studies [19-21] have been reported on neonatal and maternal vitamin D deficiency in Korea. However, no studies have reviewed vitamin D deficiency in VLBWIs. In the present study, the average vitamin D level in 188 VLBWIs was 13.4±9.3 ng/mL. In another Korean study, Shin et al. [21] observed that 668 newborns had an average 25(OH)D of 32 nmol/L (12.8 ng/mL). The present study shows that 79.8% of VLBWIs had vitamin D deficiency, and 44.1% had severe vitamin D deficiency. According to Park et al. [22], 91.7% of preterm babies were vitamin D deficient (n=278; from January 2013 to May 2015, mean gestational age of 33+5 weeks), and Ataseven et al. [8] found that 97% of preterm babies were vitamin D deficient (n=190; from October 2012 to June 2013, gestational age of 29–35 weeks). Similarly, according to Moon et al. [20], 97.4% of full-term babies were vitamin D deficient (n=122; from March 2013 to December 2014, full term). Therefore, vitamin D deficiency affects a significant proportion of both full-term and preterm babies regardless of their gestational age.
In this study, the incidence of vitamin D deficiency is lower than past studies. It is supposed that this is due to differences in characteristics of the study population, such as birth weight, gestational age, and maternal vitamin D status.
The vitamin D level was higher in the summer and in the fall than in spring or winter in this study. According to Holick [23], above 37° latitude during the months from November through February, there are marked decreases (~80%–100%) in the number of ultraviolet B photons reaching the earth’s surface.
As in the study of Burris et al. [14], we could not prove any correlation between gestational age and vitamin D level. In this study, babies with a lower birth weight were vulnerable to vitamin D deficiency, which is in accordance with the study by Morgan et al. [24] They found an inverse association between 25(OH)D concentration and low birth weight.
Unlike our study, the Park et al. [22] study found that the risk of severe vitamin D deficiency in twin preterm infants was significantly higher than that in singletons (OR, 1.993; 95% confidence interval, 1.137–3.494; P=0.016).
The duration of oxygen usage, mechanical ventilation and noninvasive oxygen usage was longer in the lower vitamin D group. Kazzi et al. [25] scored RSS on the first day of life, and formed 3 categories: mild (1.0–3.9), moderate (4.0–6.9), and severe (7.0–10.0). Post hoc comparison with mean 25(OH)D levels demonstrated significant differences (P=0.042). Also, the group with lower vitamin D levels had higher RSS (P=0.012) on the first day of life, and a higher number of babies were treated with surfactant (P=0.039).
In terms of respiratory morbidities, RDS was more frequent in preterm babies with severe vitamin D deficiency. Çetinkaya et al. [9] reported that vitamin D levels in patients with BPD were significantly lower in 132 preterm infants born at less than 32 weeks of gestation (P<0.001), and all patients with BPD had vitamin D levels <10 ng/mL. It is not clear yet why babies with vitamin D deficiency are at higher risk for respiratory morbidities. However, we can hypothesize the cause and the consequences. Babies with vitamin D deficiency had a longer duration of O2 usage; therefore BPD would subsequently occur. ROP was also influenced by the duration of oxygen treatment. Therefore, it is assumed that ROP would also occur more in the group with the lowest vitamin D.
In Table 1, maternal age was significantly higher in the lower vitamin D group. There is no other research related to this result, and further analysis is needed. In addition, some studies have shown that low maternal vitamin D predisposes children to wheezing or asthma [26,27], so the effect of vitamin D extends beyond the neonatal period to childhood and adolescence. Consequently, further studies are needed on the association between vitamin D and respiratory diseases.
The limitation of this study is that when we divided the patients into 3 groups, we did not correct for confounding factors (gestational age, birth weight, either twin or singleton, seasons of birth, maternal history, etc.). Moreover, we were unable to investigate the association between maternal serum vitamin D levels and fetal vitamin D levels because the data were not available. The World Health Organization/Food and Agriculture Organization of the United Nations recommended that nutrient intake for vitamin D in pregnant women be 200 IU/day [28]. However, because of the limited evidence to directly assess the benefit and harm of vitamin D supplementation alone in pregnancy, the use of this intervention as part of routine antenatal care is also not recommended [29]. Nevertheless, as vitamin D deficiency is associated with preterm birth [30], small-for-gestational-age infants [31], rickets [32], preeclampsia [15], and gestational DM [33], this picture is expected to improve with proper dosing. Another limitation is that this study may not be able to be generalized to other hospitals or other countries, as our study is a single-institutional study and the findings may vary according to nutritional status, race, sunlight exposure, and gestational age.
Vitamin D deficiency is a serious condition worldwide. According to the Korean National Health and Nutrition Examination Survey 2008 [34], vitamin D insufficiency was defined as 25(OH)D <20 ng/mL. The prevalence of vitamin D insufficiency was 47% in teenage boys and 64.5% in teenage girls. Efforts to support adequate maternal vitamin D intake, outdoor activity during pregnancy, and an adequate supply of vitamin D in neonates are important for preventing neonatal respiratory morbidities. Further multicenter randomized trials and long-term follow up are needed to determine the effect of vitamin D on respiratory outcomes.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.