The change of QRS duration after pulmonary valve replacement in patients with repaired tetralogy of Fallot and pulmonary regurgitation
Article information
Abstract
Purpose
This study aimed to analyze changes in QRS duration and cardiothoracic ratio (CTR) following pulmonary valve replacement (PVR) in patients with tetralogy of Fallot (TOF).
Methods
Children and adolescents who had previously undergone total repair for TOF (n=67; median age, 16 years) who required elective PVR for pulmonary regurgitation and/or right ventricular out tract obstruction were included in this study. The QRS duration and CTR were measured pre- and postoperatively and postoperative changes were evaluated.
Results
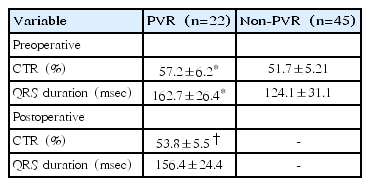
Following PVR, the CTR significantly decreased (pre-PVR 57.2%±6.2%, post-PVR 53.8%±5.5%, P=0.002). The postoperative QRS duration showed a tendency to decrease (pre-PVR 162.7±26.4 msec, post-PVR 156.4±24.4 msec, P=0.124). QRS duration was greater than 180 msec in 6 patients prior to PVR. Of these, 5 patients showed a decrease in QRS duration following PVR; QRS duration was less than 180 msec in 2 patients, and QRS duration remained greater than 180 msec in 3 patients, including 2 patients with diffuse postoperative right ventricular outflow tract hypokinesis. Six patients had coexisting arrhythmias before PVR; 2 patients, atrial tachycardia; 3 patients, premature ventricular contraction; and 1 patient, premature atrial contraction. None of the patients presented with arrhythmia following PVR.
Conclusion
The CTR and QRS duration reduced following PVR. However, QRS duration may not decrease below 180 msec after PVR, particularly in patients with right ventricular outflow tract hypokinesis. The CTR and ECG may provide additional clinical information on changes in right ventricular volume and/or pressure in these patients.
Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease, with an incidence rate of 5%–7% [1]. Characteristic anatomic features include (1) right ventricular outflow tract (RVOT) stenosis, (2) ventricular septal defect, (3) overriding aortic root, and (4) right ventricular hypertrophy [2-4].
Total correction of TOF eliminates the RVOT stenosis and closes the interventricular septal defect. Patients who underwent successful corrective surgery generally are symptomfree and show good recovery, but pulmonary regurgitation (PR) may remain after relief of RVOT stenosis [1,4]. Although PR severity may vary, severe or prolonged PR can in turn lead to RV dilation and functional decline, hypokinesis, and ventricular arrhythmia [1,5-7]. These changes require long-term regular follow-up using chest X-ray, electrocardiogram (ECG), and echocardiography. ECG and chest X-ray can show QRS duration >180 msec and increased cardiothoracic ratio (CTR), respectively.
Pulmonary valve replacement (PVR) for patients with repaired TOF improves signs of heart failure caused by chronic volume overload of the RV. It can decrease RV volume and size, eliminate the propensity for arrhythmia, and decrease the risk of sudden cardiac death. During regular follow-up after PVR, not only echocardiography but also ECG and chest X-ray may show these changes [8].
This study investigated the changes of QRS duration and CTR after PVR using ECG and chest X-ray in patients with TOF and severe PR.
Materials and methods
1. Patients
Among patients who was diagnosed with TOF prior to age 1 and underwent total correction, 67 receiving regular follow-up between January 2005 and February 2015 for PR or RVOT stenosis were included in the analysis. Enrolled patients were divided into 2 groups such as PVR group and non-PVR group according to need for PVR due to severe PR.
Severe PR were examined using echocardiography defined as abnormal pulmonary valve (distorted or absent leaflets, or annular dilation), RV enlargement, paradoxical septal motion, Color jet which fills RVOT, and/or continuous wave jet density and contour (dense laminar flow with steep deceleration slope and abrupt termination) [9,10].
Total correction of TOF involved median sternotomy, followed by patch closure of the interventricular septum through an RV or right atrial approach under cardiopulmonary bypass and circulatory arrest, as well as enlargement of the RV outflow tract and stenotic pulmonary arteries [11]. The timing of PVR for severe PR was determined via 2-dimensional echocardiography and cardiac catheterization. For male patients, mechanical valve was chosen first.
A 12-lead ECG was performed preoperatively and 1 year postoperatively, and the QRS duration was analyzed. The ECG was performed at a paper speed of 25 mm/sec, and QRS duration was defined as the interval from the beginning of the Q wave to the end of the S wave, corresponding to the time from ventricular depolarization to activation of all parts of the heart. The longest QRS duration was used for this analysis.
The CTR was calculated from the chest X-ray performed at the time of admission for PVR and 1 year postoperatively.
Patient information was retrieved and evaluated with the approval of the medical records department and Institutional Review Board at Kyungpook National University Hospital (approval number: 2016-11-019).
2. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA), and the results were indicated as mean±standard deviation or median. Comparative analysis was performed using Student t test, and a P value of less than 0.05 was defined as statistically significant.
Results
1. Demographic data
Of 67 patients analyzed, 22 underwent PVR (Table 1). Sixteen patients (16 of 22, 73%) needed transannular (TAP) patch insertion at the time of initial TOF correction. Patients were followed up for a median of 14.5 years (range, 6–22 years) from the time of initial TOF correction to PVR. The median age at the time of PVR was 16 years (range, 9–30 years). Eleven patients (11 of 22, 50%) showed RV hypertrophy and diffuse RV hypokinesis on pre-PVR computed tomography and echocardiography, and 1 had an RVOT aneurysm.
The main reason for PVR was severe PR in 20 and severe PR accompanied by pulmonary stenosis (PS) in 2. Mechanical and tissue valve were used for PVR in 8 (8 of 22, 36%) and 14 (14 of 22, 64%), respectively. Eleven patients (11 of 22, 50%) needed addition procedures with PVR; peripheral pulmonary angioplasty in 6, tricuspid valve repair in 4, and maze operation for atrial tachycardia (AT) in 1. The median duration of follow-up after PVR was 3.9 years (range, 0.9–8.3 years).
The median age of the 45 patients who did not undergo PVR was 13 years at the latest follow-up (range, 8–23). There were 23 patients (23 of 45, 51%) with TAP at the time of initial TOF correction. The median duration of follow-up after initial total TOF correction was 11.2 years (range, 6.7–23 years).
2. Change in CTR
CTR in the PVR and non-PVR groups is shown in Table 2. CTR was significantly different between the PVR and non-PVR group (P=0.001), and reduced from pre-PVR to post-PVR (P=0.002).
3. Change in QRS duration
QRS duration was significantly different between the PVR and no-PVR group (Table 2) (P=0.001). Compared with pre-PVR QRS duration, post-PVR QRS duration was decreased, but not significantly (P=0.124).
There were 6 patients with pre-PVR QRS duration >180 msec (Table 3). Among them, 5 patients showed reduction of QRS duration after PVR; post-PVR QRS duration <180 msec in 2 and post-PVR QRS duration >180 msec in 3. There was 1 patient with pre-PVR and post-PVR QRS duration >180 msec without change after PVR. Of the 4 patients with post-PVR QRS duration >180 msec, 2 had diffuse RVOT hypokinesis postoperatively.
There were 16 patients with pre-PVR QRS duration <180 msec. Among them, 6 patients showed reduction of QRS duration after PVR and had post-PVR QRS duration <180 msec. Although 7 patients showed increase or no change of post-PVR QRS duration, they had post-PVR QRS duration <180 msec. Three patients with pre-PVR QRS duration <180 msec showed post-PVR QRS duration >180 msec. They already had trifascicular block, and needed redo-PVR or concurrent pulmonary angioplasty at the time of PVR.
4. Change in arrhythmias
Six patients showed coexisting arrhythmias before PVR. There were AT in 2, premature ventricular contractions (PVCs) in 3, and nonconducted premature atrial contractions (PACs) and left ventricular systolic dysfunction in 1.
One patient with PVC showed pre-PVR QRS duration > 180 msec and post-PVR QRS duration <180 msec. Four patients (AT in 2 and PVC in 2) showed pre-PVR QRS duration <180 msec and reduction of post-PVR QRS duration. One patient with PAC did not show any change between pre-PVR and post-PVR.
There was no patient with arrhythmia confirmed by ECG after PVR, and no reported cases of arrhythmia recurrence by holter monitoring and symptoms, chest pain, or additional surgery during follow-up.
Discussion
We hypothesized that increased RV volume in repaired TOF and severe PR would decrease after PVR, and CTR on chest X-ray and QRS duration on ECG would reflect these changes. From the present study, we found that post-PVR CTR on chest X-ray showed significant decrease. In addition, PVR could make reduction of QRS duration for patients with pre-PVR QRS duration >180 msec and improvement of arrhythmia, although some patients still showed post-PVR QRS duration >180 msec.
ECG can be a simple yet clinically relevant indicator for RV function and long-term prognosis in patients who have undergone TOF correction. In previous studies, QRS duration on ECG was related to the degree of RV hypertrophy, arrhythmia, and sudden death. In particular, QRS duration >180 msec was associated with ventricular tachycardia, RV dilatation, and sudden death [1,4,10]. Moreover, patients who underwent surgery for TOF showed increased long-term mortality due to ventricular arrhythmia [12].
Previous studies reported that patients with severe PR who underwent surgical TOF correction showed post-PVR improvement in arrhythmia as well as a decrease in QRS duration >180 msec [13,14]. However, recent studies show that the relationship between PVR and QRS duration is not clear [5,15,16]. QRS duration >180 msec prior to PVR that did not decrease after PVR was associated with symptomatic heart failure, repeat PVR, ventricular arrhythmia, and death [17]. The present study showed that PVR was related with decrease in post-PVR QRS duration in 50% of patients and resolution of arrhythmia. However, 67% of patients with pre-PVR QRS duration >180 msec had still post-PVR QRS duration >180 msec. They showed pre-PVR CTR greater than 60%, or decreased cardiac function associated with ventricular arrhythmia and likely did not show normal recovery of RV function or volume postoperatively.
The results of the present and previous studies indicate that despite elimination of PR and decreased RV volume loading after PVR, severe and prolonged PR leads to irreversible damage to RV myocytes due to volume overload and inhibits recovery of RV function to a normal level.
Another study proposed that prolonged QRS duration is related to infundibular disease due to local damage from TOF, rather than RV dysfunction [18]. A damaged RVOT affects postoperative ventricular remodeling, RV function, and dyssynchrony of ventricular contraction and causes heterogeneous conduction. This can lead to persistent risk of arrhythmia in TOF patients with cardiomyocyte damage and tissue remodeling due to preoperative cyanosis, ventricular pressure, or volume loading [15,16,19]. In other words, PVR plays a role in decreasing RV volume loading but does not alleviate RVOT pathology, therefore may not decrease QRS duration [5,16,19,20]. In the present study, patients who showed increase or no change of post-PVR QRS duration underwent infundibulectomy and TAP. They showed RV hypertrophy, severe PR, and diffuse RV hypokinesis on preoperative computed tomography and echocardiography, and 1 had an RVOT aneurysm. Therefore, current TOF corrective surgery is focused on the RV infundibulum and pulmonary valve preservation. This is probably because these are important factors leading to arrhythmia and sudden death, as shown in this study.
The limitations of this study include the small sample size and retrospective study design. We did not perform RV volume measurement using cardiac magnetic resonance imaging (MRI) or 3-dimensional echocardiography routinely. It was not able to compare results between MRI or echocardiogram and CTR and ECG. The possibility of bias due to the timing of PVR after TOF correction and remnant pathology or symptoms cannot be ignored.
In conclusion, CTR and QRS duration tend to reduce after PVR. However, the absence of a reduction in QRS duration <180 msec after PVR may be occurred, especially in patients with RVOT hypokinesis. Although cardiac MRI is preferred for the analysis of RV volume and function in patients with repaired TOF, CTR on chest X-ray and QRS duration on 12 lead ECG are easily evaluated in clinical practice, and these may provide additional clinical information reflecting changes in RV volume and/or pressure.
Notes
No potential conflict of interest relevant to this article was reported.