Familial Mediterranean fever presenting as fever of unknown origin in Korea
Article information
Abstract
Familial Mediterranean fever (FMF) is the most common Mendelian autoinflammatory disease, characterized by uncontrolled activation of the innate immune system that manifests as recurrent brief fever and polyserositis (e.g., peritonitis, pleuritic, and arthritis). FMF is caused by autosomal recessive mutations of the Mediterranean fever gene, MEFV which encodes the pyrin protein. Although FMF predominantly affects people from Mediterranean and Middle Eastern ethnic origins, 3 cases of FMF have been reported in Korea since 2012. We report another case of FMF in Korea in which the patient presented with a month-long fever without serositis. After treatment with colchicine was initiated, the patient’s symptoms quickly subsided. The response to colchicine was helpful for diagnosis. We compare the FMF genotypes in Korea with in other countries. Studying FMF cases in Korea will help establish the best MEFV exons to use for screening and diagnosis of Korean FMF.
Introduction
Familial Mediterranean fever (FMF) is a systemic autoinflammatory disease characterized by recurrent fever with polyserositis such as peritonitis, pleuritis and arthritis1). FMF is caused by autosomal recessive mutations of the MEFV gene, which encodes the pyrin (or marenostrin) protein1). Mutated pyrin increases interleukin-1β activation and accentuates innate immune activation2). FMF mainly affects people of Mediterranean and Middle Eastern origin, in whom carrier frequencies for FMF-related MEFV gene mutations are as high as 1 in three3). However, some FMF cases have been reported in other ethnic groups living far from the Mediterranean. Clinical manifestations of FMF are not pathognomonic and timely diagnosis can be difficult, especially in areas where FMF is not prevalent. This difficulty in diagnosis could contribute to the development of renal amyloidosis, a devastating complication of FMF14).
Since the first report of a patient with FMF in Korea in 2012 year4), 2 more cases of FMF have been reported56). Diagnoses were delayed and consequently, 2 of the patients had severe complications45). Here, we report another case of FMF in Korea: the patient presented with fever only for 1 month without other manifestations.
Case report
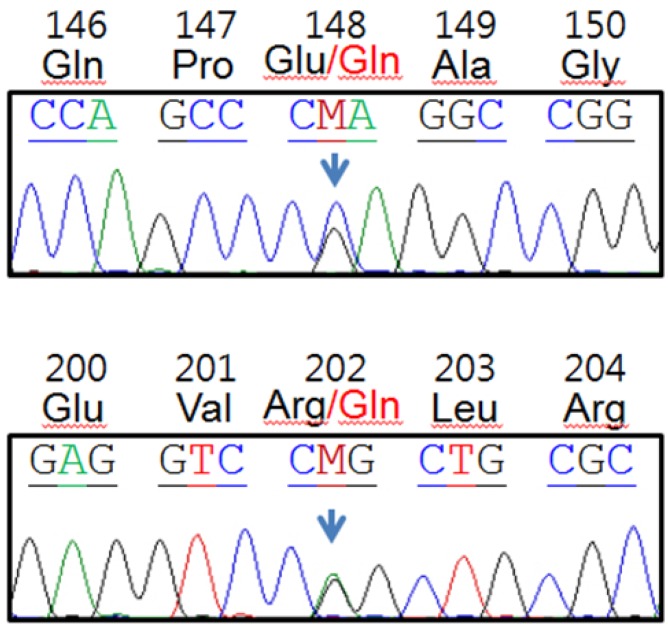
A 17-year-old boy was admitted to our hospital on August 19, 2013 for a 38℃ fever that started 7 days before admission, mainly during the night. The patient recalled a similar attack 5 months before that lasted for 7 days without treatment. The patient had no past medical or surgical history. His parents were not consanguineous and family history was not specific. On admission, he had a fever of 39.3℃. He had no skin, eye, chest, abdominal or musculoskeletal system symptoms. Physical examination did not reveal abnormalities, such as rash. Laboratory findings on admission were not remarkable except for high C-reactive protein (225 mg/L) and high erythrocyte sedimentation rate (67 mm/hr), both of which were sustained for the duration of febrile days (Fig. 1). To determine the cause of fever, chest and abdominal computed tomography scanning, gastrocolonoscopy, echocardiography and bone scans were performed, but all were negative. Cultures and serologic tests for infectious origin, autoimmune antibodies or tumor markers failed to reveal the cause of fever. The patient was fine during fever-free periods. We considered common causes of fever of unknown origin unlikely for the patient and suspected periodic fever syndrome. IgD was within the normal range (9 mg/dL) but serum amyloid A level was remarkably high (1,010 µg/mL). Colchicine was started at 1.2 mg/day and the fever responded from the fourth day of treatment. DNA analysis of exons of the MEFV gene, where most MEFV mutations are found, revealed 2-point mutations, resulting in E148Q and R202Q amino acid substitutions (Fig. 2). The patient is currently receiving daily colchicine without fever.
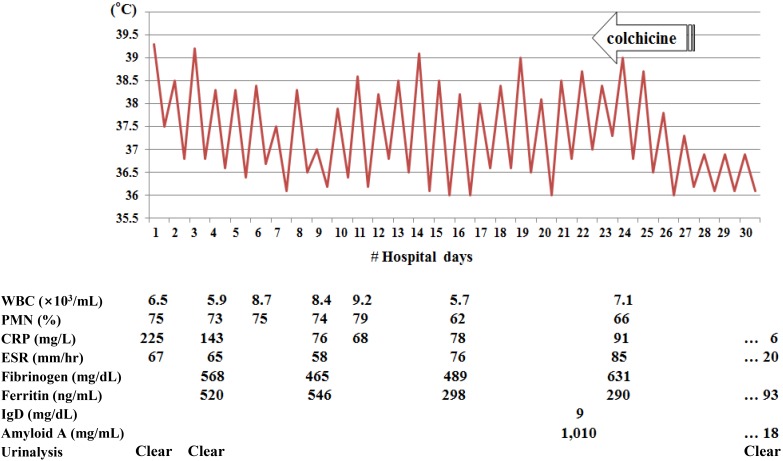
The highest and lowest body temperatures during hospitalization. Colchicine administration was initiated on the 23rd hospital day. Corresponding laboratory data are shown. WBC, white blood cells; PMN, polymorphoneutrophils; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Discussion
Diagnosis of FMF is based on typical clinical manifestations, family history and colchicine responsiveness7). According to the criteria for FMF diagnosis, fever is short, lasting between 12 hours and 3 days; the fever can be longer or shorter than specified, but not shorter than 6 hours or longer than 1 week7). While all previously reported Korean cases had typical attacks456), our patient presented with only a fever whose nature was not typical of FMF. Fever can be the only manifestation of FMF years before other symptoms appear or can occur for the entire duration of childhood8). In cases with atypical clinical manifestations and a patient with no family history such as the case described in this study the use of colchicine for diagnostic and therapeutic purposes is justified9).
Our patient had two MEFV gene mutations. The E148Q mutation is considered to be relevant to disease manifestation10) but whether R202Q is a disease-causing mutation is unclear. While allelic frequencies at codon 202 were the same in control and FMF patients in a Spanish population11), the R202Q polymorphism and FMF were strongly associated in a Turkish population12). R202Q and E148Q are reported to cause a mild change in electrochemical energy of the pyrin protein13). Gene-dosage effects generated by multiple MEFV mutations or polymorphisms in trans or in cis might contribute to altered protein function and eventually, disease development2).
MEFV gene mutation frequencies in FMF patients have been compared by region (Table 1). Mutation frequencies in Turkey and Germany are similar to frequencies in the Mediterranean basin. M694V is the most common mutation and M694I is the rarest mutation in Turkey and Germany; however the opposite is true in Japan. In Turkey and Germany, most mutations are located in exon 10 and exon 2, and rarely in exon 3; in Japan, most mutations are located in exons 2 and 10 or 3101415). Although only 4 cases of FMF have been documented in Korea, most mutations were in exons 2 and 3, and none were found in exon 10456). Although the number of Korean FMF patients is too small to draw definitive conclusions, Korean MEFV mutation frequencies might vary widely from frequencies in other countries. Studying additional FMF cases in Korea will help establish which MEFV exons should be screened to diagnose Korean FMF.

MEFV mutation frequencies in Familial Mediterranean fever patients in Korea compared with frequencies in patients from other countries
The response to colchicine in FMF was rapid and remarkable compared to the response of other periodic fever syndromes to colchicine. More than 90% of patients with typical clinical manifestations of FMF show a favorable response to colchicine regardless of the number of MEFV mutations1). Negative mutation analysis of the MEFV gene should therefore not exclude the use of colchicine.
High suspicion is required to diagnose FMF in areas where FMF is not prevalent. Doctors should, be aware of FMF and the fact that FMF-associated fever is not necessarily brief. Genetic analysis showing one or more FMF-associated MEFV mutations can help confirm diagnosis. Colchicine responsiveness may be one of the most valuable criteria for diagnosing FMF in Korea.
In conclusion, we diagnosed a 17-year-old boy with FMF based on colchicine-responsive fever and MEFV mutation analysis. For patients with recurrent and prolonged fever, FMF should be considered after excluding the most common causes of fever of unknown origin. Targeted exon analysis of the MEFV gene and colchicine trials should be considered for the early recognition of FMF and prevention of further development of renal amyloidosis.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.