Long-term prenatal stress increases susceptibility of N-methyl-D-aspartic acid-induced spasms in infant rats
Article information
Abstract
Purpose
Infantile spasms, also known as West syndrome, is an age-specific epileptic seizure. Most patients with this condition also exhibit delayed development. This study aimed to determine the effect of long-term prenatal stress on susceptibility to infantile spasms.
Methods
We subjected pregnant rats to acute or chronic immobilization stress. Resulting offspring received N-methyl-D-aspartic acid (15 mg/kg, intraperitoneally) on postnatal day 15, and their behaviors were observed 75 minutes after injection. The expression of KCC2 and GAD67 was also determined using immunohistochemistry.
Results
Exposure to long-term prenatal stress increased the frequency of spasms and decreased the latency to onset of spasms compared with offspring exposed to short-term prenatal stress. Expression of KCC2 and GAD67 also decreased in the group exposed to long-term prenatal stress compared with the group exposed to short-term prenatal stress.
Conclusion
Our study suggests that exposure to long-term prenatal stress results in increased susceptibility to seizures.
Introduction
Infantile spasms (IS), also known as West syndrome, is an age-specific epileptic seizure and the majority of patients with this condition also exhibit symptoms of delayed development. West syndrome is characterized by (1) a specific electroencephalographic (EEG) abnormality called hypsarrhythmia, (2) epileptic spasms with truncal flexion, and (3) psychomotor deterioration.1) Although this catastrophic epilepsy was first identified more than 160 years ago, its cause and mechanism are insufficiently understood.2)
Stress is a constant part of life. Thus, the damage to the baby may be substantial if the pregnant woman's stress continues throughout the pregnancy. Primarily, prenatal stress has been demonstrated to cause central nervous system disorders, such as alterations in the hypothalamic-adrenal-pituitary (HPA) axis3) and epilepsy4) in infants.
Numerous neuropsychiatric disorders are known to be caused by the dysfunction of the gamma-aminobutyric acid (GABA)ergic signaling system, including, autism spectrum disorder,5) schizophrenia,6) and epilepsy.78) The excitatory/inhibitory shift is interrupted during cerebral development due to prenatal stress and this may result in an increased susceptibility to spasms. Baek et al.8) have previously demonstrated that prenatal exposure to acute immobilization stress (AIS) leads to increased susceptibility to IS. However, considering the diversity of stress, different responses may be expected depending on the degree of stress.
Thus, we investigate the effect of long-term prenatal stress on the GABAergic signaling system by using acute and chronic immobilization stress (CIS) models.
Materials and methods
1. Animals
Pregnant Sprague-Dawley rats purchased from Samtako Bio Korea (Osan, Korea) were housed at a temperature of 23℃ under a controlled 12:12 light: dark cycle with ad libitum food and water. All experiments were carried out with the approval of the Animal Care and Use Committee at the Chungnam National University (CNU-00789) and were consistent with the ethical guidelines of the National Institutes of Health.
The protocol for AIS used was adapted from previous publications.8910) Pregnant rats were subjected to AIS on gestational day 15 (G15) by taping four limbs to metal mounts attached to a board for 45 minutes at 09:00 and 18:00 hour under bright light (Fig. 1A).
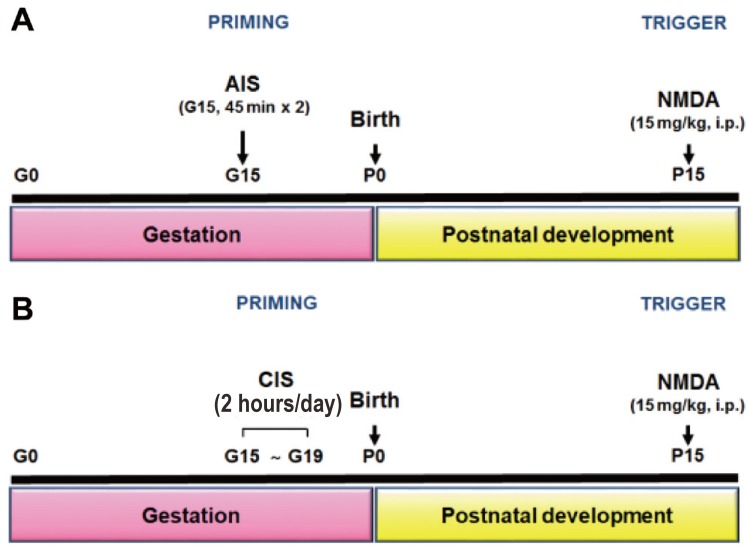
(A, B) N-methyl-D-aspartic acid (NMDA)-induced spasms in the offspring of rats exposed to acute immobilization stress (AIS) or chronic immobilization stress (CIS) during gestation. Timeline of experiments from prenatal exposure to AIS (A) or CIS (B) to postnatal NMDA-triggered spasms. i.p., intraperitoneally.
In the case of CIS, the following protocol was established. Pregnant rats were exposed to CIS, once each day for 2 hours, from day 15–19 of gestation.11) To prevent the habituation of the animals to the daily exposure to these procedures, restraint time was randomly shifted to different times (09:00 to 18:00 hour) of the day. For immobilization stress, the head fix restrainer (#JD-R-14 Φ 60×180mm, Jeung Do Bio & Plant CO., LTD, Seoul, Korea) was used (Fig. 1B).
Fifteen postnatal day 15 (P15) SD rats were divided into three groups: (1) control (n=5), (2) AIS offspring (n=5), and (3) CIS offspring group (n=5) received 15-mg/kg NMDA administration on P15. After NMDA administrations, the offspring in each group were observed for 75 minutes. The onset of a spasm was defined by high degree of flexion (i.e., hand and trunk flexion, forelimb, hind limb, and hip flexion). We observed the latency from NMDA administration to the onset of the spasm, and the total number of spasms during observational period.
2. Immunohistochemistry
Five animals per group were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally) and perfused transcardially with heparinized phosphate-buffered saline (PBS) followed by perfusion with 4% paraformaldehyde in PBS. Each brains were removed, immersed in the same fixative overnight, and then cryoprotected in 10%, 20%, and 30% sucrose serially. The brains were then embedded in Frozen Section Compound (#3801480, Leica Microsystems, Nussloch, Germany) and frozen in 2-methyl butane rapidly, which had been precooled to its freezing point with liquid nitrogen. Frozen coronal sections (35 µm thick) were obtained using a Leica cryostat (Leica Microsystems, Nussloch, Germany). Parallel free-floating sections were subjected to endogenous peroxidase blocking with 1% H2O2 in PBS, followed by treatment with a blocking buffer (1% fetal bovine serum in PBS and 0.3% Triton X-100) for 30 minutes and incubation with glutamic acid decarboxylase isoform 67 (GAD67, 1:400; #MAB5406, Millipore, Billerica, MA, USA) and K+/Cl− cotransporter (KCC2, 1:200; #07-432, Millipore, Billerica, MA, USA) primary antibodies. Immunohistochemical staining of the tissue sections for KCC2 and GAD67 were performed using the avidin-biotin peroxidase complex method, which has been described previously.10) After washing with PBS, the tissues were exposed to biotinylated anti-rabbit/mouse IgG and streptavidin peroxidase complex (Vector Laboratories, Burlingame, CA, USA). Immunostaining was visualized with diaminobenzidine (DAB) and mounted using Polymount (Polysciences, Inc., Warrington, PA, USA).
3. Statistical analysis
The NIH image program (ImageJ, National Institute Health, Bethesda, MD, USA) was used to assess the immunohistochemical signals quantitatively with densitometric measurements. The quantitative data were then analyzed using nonparametric 1-way analysis of variance. The Newman-Keuls method was used for post hoc analysis. A P value less than 0.05 was considered statistically significant. All statistical analyses were conducted using the Prism 5 program (GraphPad, San Diego, CA, USA).
Results
1. NMDA-induced epileptic spasms
In this study, we used a rat model of prenatal exposure to AIS and CIS as well as postnatal NMDA-triggered spasms. After NMDA administration, the phenotype of the spasms that were observed in a P15 rat was characterized by a continuously twisting tail and a high degree of flexion. We observed that the NMDA-triggered spasms occurred earlier, more frequently, and more intensely in infant rats (P15) that were exposed to prenatal stress (Fig. 2). The frequency of spasms (mean±standard deviation [SD], times/hr) was significantly increased in infant rats exposed to either prenatal AIS (119.0±15.0) or CIS (202.3±88.6) compared to rats in the control (46.0±3.6) group (Fig. 2A). In addition, the latency to the onset of spasms (mean±SD, seconds) was significantly decreased in the prenatally AIS (826.7±67.3) and CIS (817.0±97.5) exposed rats compared with controls (1,205.3±170.4) (Fig. 2B).
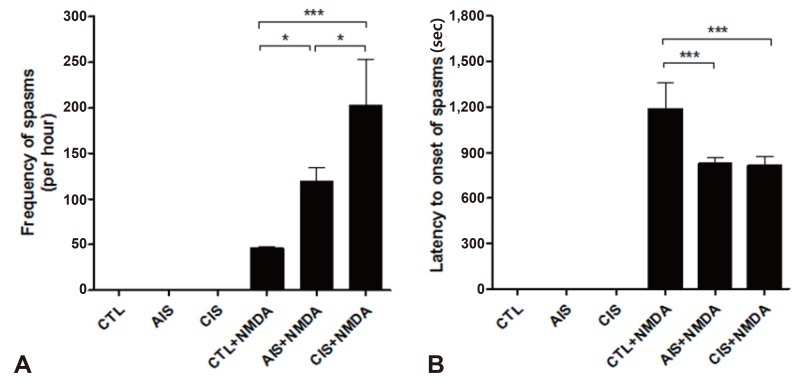
Prenatal stress accelerates the onset of N-methyl-D-aspartic acid (NMDA)-induced spasms and increases their frequency. (A, B) The frequency (per hour) and latency (seconds) to the onset of flexion spasms induced by intraperitoneal NMDA were measured in both control and treated rats on postnatal day 15. The frequency (per hour) and latency (seconds) to the onset of spasms were increased in offspring prenatally exposed to acute immobilization stress or chronic immobilization stress (*P<0.05, ***P<0.001). CTL, control; AIS, acute immobilization stress; CIS, chronic immobilization stress.
2. Decreased expression of KCC2
We examined the expression of KCC2 following prenatal exposure to AIS or CIS (Fig. 3). In the control group, KCC2 expression increased as expected as a function of age. In both prenatal-stressed groups, KCC2 was expressed at significantly lower levels than the control group in cortical layers II and III on P15. The density of KCC2 of prenatally stressed groups was also decreased in immunohistochemical staining analysis (Fig. 3M).
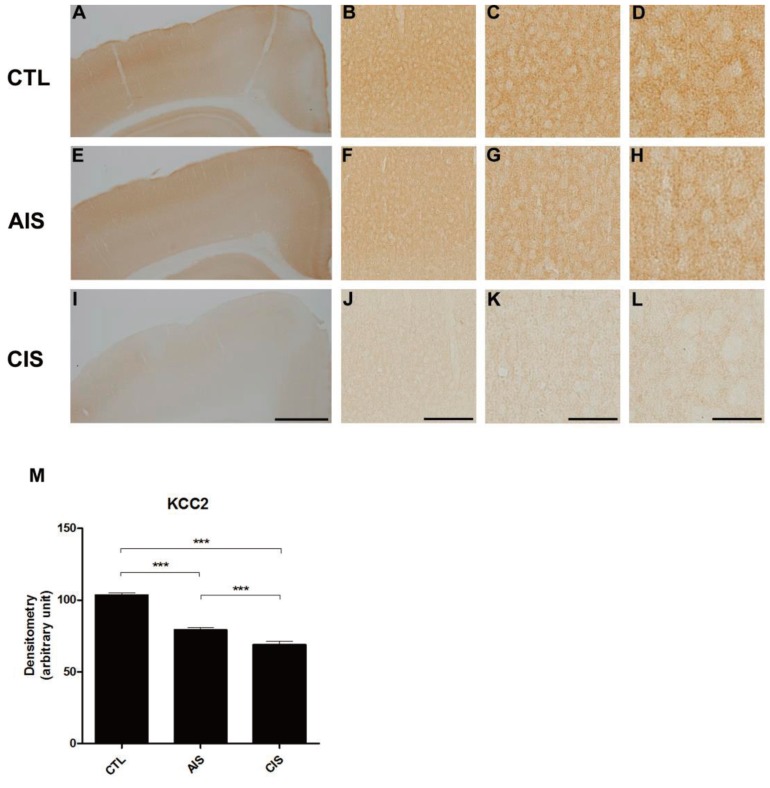
After birth, offspring show a decrease in the expression of KCC2 protein in the medial cortex due to prenatal exposure to acute immobilization stress (AIS) or chronic immobilization stress (CIS) in immunohistochemistry. (A, E, I) Representative images showing the medial cortex in control (A), AIS (E), and CIS (I) rats. Scale bars: 2 mm. (B, F, J) Representative photomicrographs showing medial cortex layers II-III in control (B), AIS (F), and CIS (J) rats. Scale bars: 800 µm. (C, G, K) Representative photomicrographs showing medial cortex layers II-III in control (C), AIS (G), and CIS (K) rats. Scale bars: 400 µm. (D, H, L) Representative photomicrographs showing medial cortex layers II-III in control (D), AIS (H), and CIS (L) rats. Scale bars: 200 µm. (M) A histogram shows a significant decrease in the expression of KCC2 in the brain when rats are exposed prenatally to either AIS or CIS (***P<0.001).
3. Decreased expression of GAD67+cells
We checked the expression of GAD67 following prenatal exposure to AIS and CIS (Fig. 4). In the control group, GAD67 expression developed as expected as a function of age. In both prenatal-stressed groups, GAD67 was expressed at significantly lower levels than the control group in cortical layers II and III in P15 rats. It demonstrated that the density of GAD67 was decreased in the prenatally stressed groups (Fig. 4M).
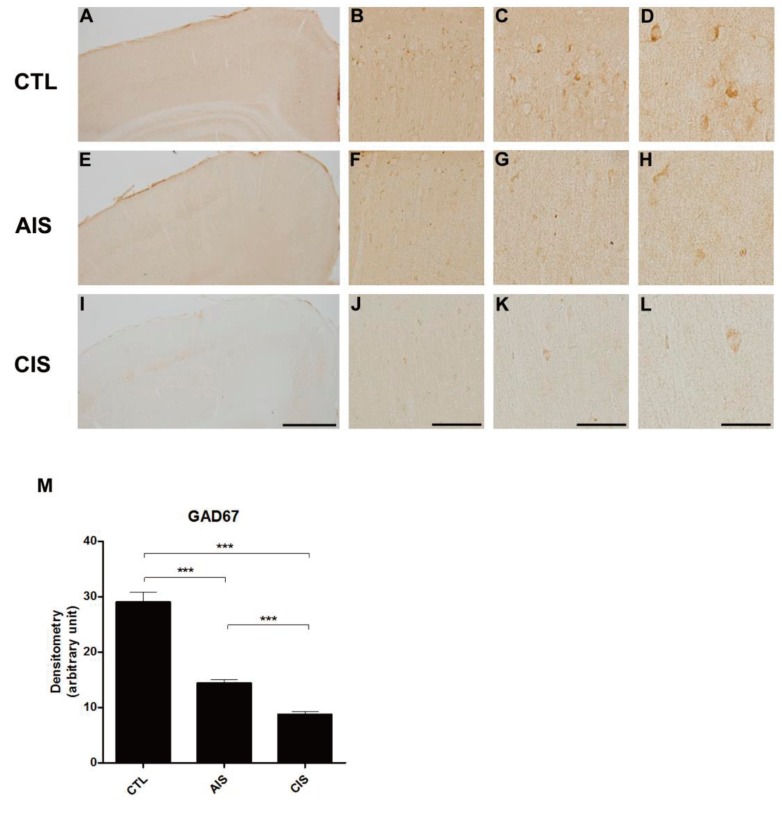
Prenatal acute immobilization stress (AIS) or chronic immobilization stress (CIS) reduces GAD67 expression in the medial cortex in immunohistochemistry. (A, E, I) Representative images showing the medial cortex in control (A), AIS (E), and CIS (I) rats. Scale bars: 2 mm. (B, F, J) Representative photomicrographs showing medial cortex layers II-III in control (B), AIS (F), and CIS (J) rats. Scale bars: 800 µm. (C, G, K) Representative photomicrographs showing medial cortex layers II-III in control (C), AIS (G), and CIS (K) rats. Scale bars: 400 µm. (D, H, L) Representative photomicrographs showing medial cortex layers II-III in control (D), AIS (H), and CIS (L) rats. Scale bars: 200 µm. (M) Quantification of the density of GAD67 immunoreactivity (***P<0.001). GAD67 density was significantly decreased in medial cortex layers II–III of rats that were prenatally exposed to AIS or CIS.
Discussion
In this study, we have demonstrated that rats exposed to long-term prenatal stress show an increased susceptibility to IS compared to control rats and rats exposed to short-term prenatal stress. Rats in the CIS group were observed to experience a greater number of NMDA-induced spasms compared to rats in the AIS group. The offspring of rats exposed to prenatal stress presented with lower protein expression levels of KCC2 and GAD67 compared to the offspring of rats in the control group. Additionally, the expression of these proteins was significantly decreased in the medial cortex of the CIS group compared to rats in the AIS group. These results suggest that exposure to long-term prenatal stress increases the susceptibility to IS compared to exposure to short-term prenatal stress.
IS are a form of epileptic encephalopathy that occurs in early childhood. In a lot of the cases, patients do not receive adequate medical therapy and this is usually responsible for the observed developmental delay.12) Understanding the cryptogenic etiology of this condition remains a challenge; however, there are various hypothesis currently being explored. Finding the cause of a seizure is important in deciding the target of treatment. Thus, studies aimed at understanding the mechanism underlying IS also contribute to research aimed at understanding developmental delay.
It is generally known that one of the causes of IS is HPA axis dysregulation which results in the upregulation of corticosterone. Prenatal stress is also known to result in HPA axis dysfunction. There are various stress protocols that can be used, however for the purpose of our experiments we use either steroid injections or immobilization stress. In a previous study,8) we observed NMDA-induced spasms after AIS in infant rats. In this study, we measured NMDA-induced spasms after both CIS and AIS in infant rats. CIS has been previously studied as a model of prenatal stress and rats exposed to this form of prenatal stress displayed various neurologic disorders such as depression and anxiety.36)
We observed that prenatal exposure to CIS, coupled with the induction of postnatal NMDA-triggered spasms, increased the frequency and decreased the latency of spasms compared to control conditions. Thus, we determined that seizure susceptibility is increased in rats exposed to the CIS model of prenatal stress compared to rats exposed to the AIS model of stress. It has previously been confirmed that in rats exposed to the AIS model of prenatal stress, there was a decrease in the expression of KCC2 expression as well as an increase in Cl- concentration in GABAergic neurons. This results in the depolarization and overexcitability of GABAergic neurons. Similarly, previous studies using patch clamp observed an increase in the resting membrane potential voltage as well as a high depolarization voltage in accordance with GABA response, leading to an increase in susceptibility to spasms. An exact amount of stress required has not be directly quantified; however, when individuals are exposed to a certain threshold of stress, the altered state of the membrane potential intensifies the susceptibility to spasms. The observed reduction in the expression of KCC2 and GAD67 cells in rats prenatally exposed to CIS supports the hypothesis that GABA response and membrane potential are relevant to mediating highly stressful situation.
One of the limitations of this study is that the animal models used do not voluntarily experience seizures. However, in other studies using a similar model, results from EEG and drug response tests were found to be appropriate for IS.813) Another limitation is that it is difficult to quantify stress accurately. In fact, observational studies in human subjects in Denmark failed to observe a significant correlation between prenatal stress and stress-related seizures in early life.14) In this study, the significant stress experienced by the mother was limited to the death of her husband, parents, or sibling. However, there are many kinds of stress that individuals experience in their daily lives. To adequately understand the association between prenatal stress and stress-induced seizures, a reliable method to quantify different forms of stress by type, intensity and duration must be developed. In this regard, it is important to confirm the susceptibility to the seizure by altering only the intensity of the bodily stress in the animal model, while keeping other conditions constant.
Although AIS was previously known to be associated with seizures, the relevance of CIS to seizures was uncertain. Ahmadzadeh et al.15) reported that short-term prenatal stress increases susceptibility of pilocarpine-induced seizure. It has been investigated by many researchers that depression,1617) memory impairment,18) and even schizophrenia19) are associated with chronic prenatal stress. Despite the above studies, the correlation between CIS and seizures was not clear. In this study, we found that chronic prenatal stress increases susceptibility of seizure in offsprings.
In conclusion, we have demonstrated that exposure to long-term prenatal stress increases the susceptibility to spasms. This suggests that long-term prenatal stress also results in the dysfunction of the GABAergic signaling system. Altering the GABAergic signaling system affects the excitation/inhibition balance, and this may increase the susceptibility to seizures.
Acknowledgments
This study was supported by a 2015 research grant from the Korean Pediatric Society (Kyun-Il Award). This work was supported by a research fund from the Chungnam National University (2014-0656-01). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (grant number: 2015R1C1A1A01052351).
