Cardiac function associated with home ventilator care in Duchenne muscular dystrophy
Article information
Abstract
Purpose
Cardiomyopathy is becoming the leading cause of death in patients with Duchenne muscular dystrophy because mechanically assisted lung ventilation and assisted coughing have helped resolve respiratory complications. To clarify cardiopulmonary function, we compared cardiac function between the home ventilator-assisted and non-ventilator-assisted groups.
Methods
We retrospectively reviewed patients with Duchenne muscular dystrophy from January 2010 to March 2016 at Gangnam Severance Hospital. Demographic characteristics, pulmonary function, and echocardiography data were investigated.
Results
Fifty-four patients with Duchenne muscular dystrophy were divided into 2 groups: home ventilator-assisted and non-ventilator-assisted. The patients in the home ventilator group were older (16.25±1.85 years) than those in the nonventilator group (14.73±1.36 years) (P=0.001). Height, weight, and body surface area did not differ significantly between groups. The home ventilator group had a lower seated functional vital capacity (1,038±620.41 mL) than the nonventilator group (1,455±603.12 mL). Mean left ventricular ejection fraction and fractional shortening were greater in the home ventilator group, but the data did not show any statistical difference. The early ventricular filling velocity/late ventricular filling velocity ratio (1.7±0.44) was lower in the home ventilator group than in the nonventilator group (2.02±0.62). The mitral valve annular systolic velocity was higher in the home ventilator group (estimated β, 1.06; standard error, 0.48). Patients with Duchenne muscular dystrophy on a ventilator may have better systolic and diastolic cardiac functions.
Conclusion
Noninvasive ventilator assistance can help preserve cardiac function. Therefore, early utilization of noninvasive ventilation or oxygen may positively influence cardiac function in patients with Duchenne muscular dystrophy.
Introduction
Duchenne muscular dystrophy (DMD; Online Mendelian Inheritance in Man [OMIM] reference 310200) is an X-linked disease that affects 1 in 3,600–6,000 live male births.1) DMD is caused by a mutation in the dystrophin gene on chromosome Xp21.1, which contains 79 exons of coding sequence and 2.5 Mb of DNA. The absence of expressed dystrophin leads to disruption of the cytoskeleton and loss of components of the cytoskeleton.2)
Patients with DMD are rarely symptomatic at birth and often show normal development until age 2 years. Thereafter, they show progressive muscle weakness, such as Gower's sign, and lose ambulation at 7–12 years old. Untreated patients' survival averages 20 years. The most common causes of mortality are respiratory and cardiac complications. Patients with DMD are at risk of respiratory complications as their condition deteriorates because of progressive loss of respiratory muscle strength.3) Advances in the respiratory care of patients with DMD have improved their prognosis. Nocturnal home ventilators and mechanically assisted coughing lead to improved survival of patients with DMD.45) The American Thoracic Society has published a statement regarding the respiratory care of patients with DMD, including evaluation and management (i.e., respiratory muscle training, mechanical ventilation, corticosteroids, and end-of-life care).6) These improvements in respiratory care make cardiac complications the leading cause of death in patients with DMD.7891011)
Cardiac dysfunction in patients with DMD often manifests as cardiomyopathy or cardiac arrhythmia. Progression of cardiomyopathy is the major cause of mortality. Cardiomyopathy can occur at any age but often occurs around 14–15 years.712) Angiotensinconverting enzyme (ACE) inhibitors, angiotensin receptor blockers, beta-blockers, or aldosterone antagonists are often used for management of Duchenne cardiomyopathy and show improvements in cardiac function. Oral corticosteroid treatment can delay the onset of Duchenne cardiomyopathy.13) However, appropriate pharmacological management and the optimal time to start pharmacotherapy need further study.
Baseline assessment of cardiac function is needed for patients with DMD at diagnosis, and annual cardiac assessment is recommended for patients with DMD older than 10 years. Poor treatment outcomes have been seen in patients with DMD who fail to see a cardiologist after the onset of clinical symptoms of heart failure.3)
The degree of skeletal muscle weakness does not correlate with the severity of cardiomyopathy in patients with DMD. Thus, cardiopulmonary function relation theoretically may not be definite in patients with DMD. Therefore, we compared the cardiac function of the home ventilator group with the nonventilator group of patients with Duchenne muscular dystrophy.
Materials and methods
1. Patients
This was a retrospective, single-center study. We reviewed 54 patients with DMD who visited Gangnam Severance Hospital between January 2010 and February 2016. These patients were diagnosed with DMD by DMD gene mutation, the absence of dystrophin expression on muscle biopsy, or a phenotype consistent with DMD. Their age was 10–20 years, and they were all males. We divided the 54 patients into 2 groups: the home ventilator assistance group (n=24) and the nonventilator assistance group (n=30). The home ventilator was applied to DMD patients whose overnight sleep endtidal CO2 average is higher than 40 mmHg. Among home ventilator assisted group, 21 patients needed overnight ventilator support, and 3 patients needed continuous home ventilation.
2. Echocardiographic measurement and pulmonary function measurement
Echocardiographic measurements were obtained in both groups using a Siemens model ACUSON SC2000 (Siemens Medical Solutions USA, Inc., Mountain View, CA, USA). Echocardiographic examinations were conducted according to the recommendations of the American Society of Echocardiography. On conventional echocardiographic measurements, the left ventricular ejection fraction (LVEF) was measured using the M-mode and Simpson's methods.
Pulmonary function tests are routinely performed by our pulmonary rehabilitation center at least annually. These tests were performed by acceptable spirometry. Sleep end-tidal CO2 average monitoring was done with SenTec digital monitoring system (SenTec AG group, Therwil, Switzerland).
3. Statistical analysis
All statistical analyses were made by using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). We compared all demographic, echocardiographic, and pulmonary function factors of patients with DMD by independent t tests and multiple linear regression.
Results
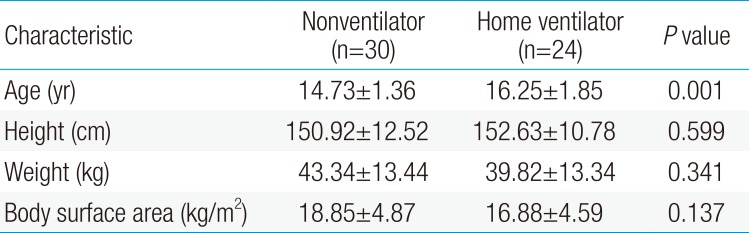
We reviewed 54 cases of DMD divided into 2 groups based on their use of home respiratory assistance devices. The demographic characteristics of the 2 groups of patients with DMD are shown in Table 1. The mean age of patients in the home ventilator assisted group (16.25±1.85 years) was higher than that in the nonventilator assisted group (14.73±1.36 years) (P<0.001). Height, weight, and body surface area was not significantly different between the 2 groups.
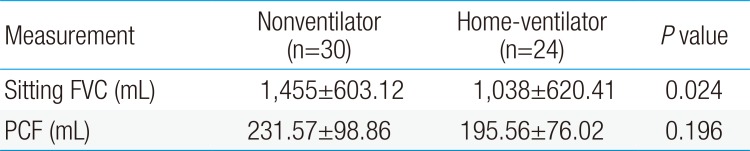
Pulmonary function analysis data of the 2 groups is shown in Table 2. The home ventilator group (1,038±620.41 mL) had a lower seated functional vital capacity (FVC) than the nonventilator group (1,455±603.12 mL). Peak cough flow did not differ between the 2 groups.
In nonventilator assisted group, 15 patients showed hypercapnia symptoms, and the most common symptoms are dyspnea and headache followed by insomnia, daytime sleepness, fatigue, and anxiety. Among them, 13 patients showed symptoms improvements after applying home ventilator.
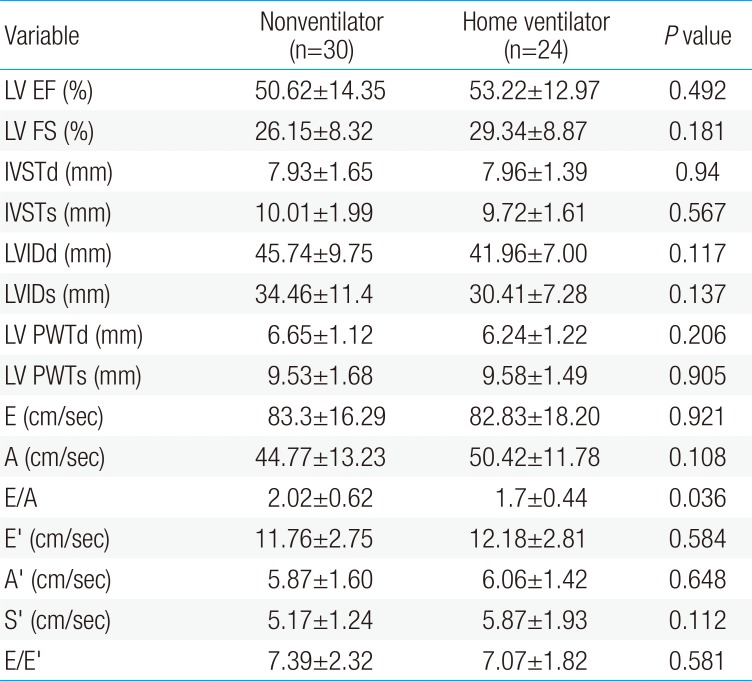
LVEF and fractional shortening (FS) showed slightly lower values in the nonventilator assistance group (ejection fraction [EF], 50.62%±14.35%; FS, 26.15%±8.32%) than in the home ventilator assistance group (EF, 53.22%±12.97%; FS, 29.34%±8.87%) without statistical difference. The LVEF of both groups was lower than 55%, compatible with heart failure according to Canadian cardiovascular society guidelines.14) Neither interventricular wall thickness nor left ventricular posterior wall thickness showed hypertrophy in either group, without any statistical difference. The left ventricle dimension in diastole and systole did not show a difference between the 2 groups, and there was no left ventricular dilatation.
According to Doppler signals, the mitral inflow velocity did not show a statistical difference. However, the early ventricular filling velocity/late ventricular filling velocity ratio (2.02±0.62) was significantly higher in the nonventilator assistance group than in the home ventilator assistance group (1.7±0.44) (P=0.03). The tissue Doppler velocities show no difference in early, late diastolic, or systolic peak mitral annuli velocity (E′, A′, S′). The E/E′ seemed to be within a reasonable range and did not show a statistical difference between the 2 groups (Table 3).
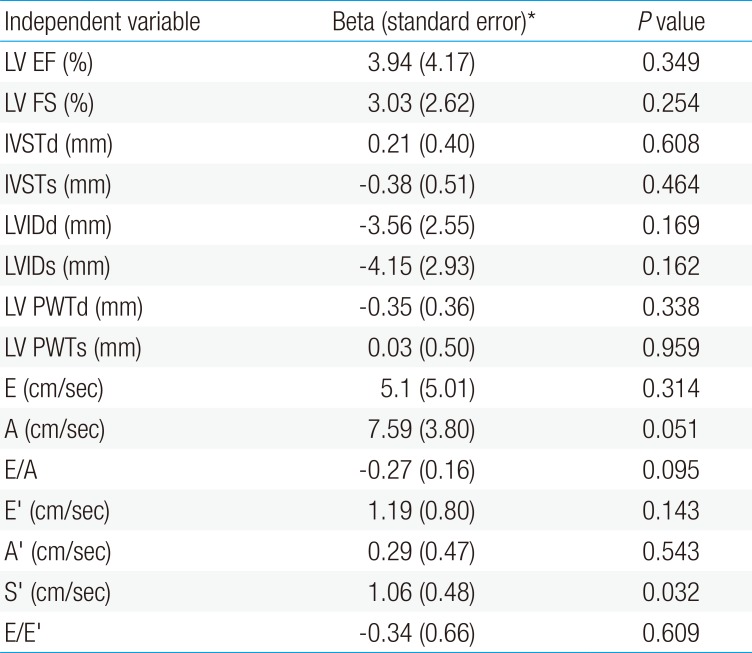
In a multiple linear regression model, tissue Doppler systolic S′ was higher in the home ventilator assistance group and lower in the nonventilator assistance group. This implied that the nonventilator assistance group demonstrated a relative decrease of the left ventricle's systolic function.15) Other conventional echocardiographic parameters did not show any statistical differences between the 2 groups (Table 4).
Discussion
In DMD, the leading causes of death are pulmonary complications and cardiomyopathy. In the second decade of life, respiratory and cardiac muscle dysfunction substantially aggravates the disease and impairs the quality of life.16) Recently, pulmonary complications have been overcome by improvements in treatment, including home nocturnal ventilators and mechanically assisted coughing,45) and patients often present with cardiomyopathy, which is becoming the leading cause of mortality in DMD. Regardless of a longer lifespan with enhanced pulmonary function, there is a consensus that the degree of cardiomyopathy and ventricular dysfunction does not well correlate with the degree of pulmonary dysfunction.1617)
In this study, we reviewed 54 patients who were diagnosed with DMD to evaluate the relationship between their cardiac function and pulmonary function.
In terms of cardiac function, usually older patients would be expected to have worse cardiac functions. However, the older patients in the home ventilator group showed no inferiority of ventricular function. Moreover, the home ventilator assisted group had better diastolic and systolic function than the nonventilator assisted group, even though home ventilator assisted patients were older. Our results are not compatible with the previous consensus that older patients with DMD often have more cardiac dysfunction than younger patients with DMD.12) Notably, patients in the home ventilator assisted group showed superior diastolic function although they were older. This superior result can be attributed to better respiratory support with oxygen.
Recently Mehmood et al.18) discussed that right ventricle ejection fraction showed a positive relationship with FVC. This means that even though skeletal muscle weakness does not correlate with smooth muscle weakness, noninvasive ventilation assistance can positively influence cardiac function by decreasing the cardiac burden.
Patients with DMD having hypoventilation symptoms such as dyspnea, fatigue, or sleep dysfunction or having a decreased FVC (lower than 1.25 L) are recommended to undergo an assessment of gas exchange during sleep. Patients whose sleep end-tidal CO2 averages higher than 40 mmHg with or without desaturation are recommended to start noninvasive ventilator support.3619) When we started noninvasive home ventilator support, we usually applied bilateral positive airway pressure mode or assisted control ventilation mode. And after that, we changed setting for individual patient.
And after applying home ventilator, many patients showed symptoms improvements. So it is also important that not just overnight sleep end-tidal CO2 average, clinical symptoms are important because home ventilator can make better quality of life.
This study had some limitations. This was a retrospective, single-center study. Moreover, the 2 groups had differing demographic factors in terms of age, although other demographic factors were not different between the 2 groups. Additionally, we did not review data regarding corticosteroid use and cardiac medication (angiotensin-converting-enzyme inhibitor, angiotensin II receptor blocker, beta blocker, etc.). There may have been a selection bias of patients with advanced DMD.
Patients with DMD show progressive cardiomyopathy in adolescence or young adulthood. The selective involvement of the heart and the variable degree of the cardiomyopathy should be further explored.20) Although caution should be used with supplemental oxygenation therapy so as not to subtly suppress the respiratory drive and exacerbate hypercapnia,3) the utilization of noninvasive ventilation or oxygen can benefit the cardiac function in patients with DMD.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.